Solutions and States of Matter - SAT Subject Test in Chemistry
Card 0 of 3
30 mL of 1.0 M  solution is diluted with water to a volume of 3 L. What is the new concentration of the solution?
solution is diluted with water to a volume of 3 L. What is the new concentration of the solution?
30 mL of 1.0 M 
Recall that  Thus, the volume of the solution increased from 30 mL to 3000 mL, which is a factor of 100. Thus, the new concentration must be 100 times less than the original concentration, and
Thus, the volume of the solution increased from 30 mL to 3000 mL, which is a factor of 100. Thus, the new concentration must be 100 times less than the original concentration, and  .
.
Recall that 

Compare your answer with the correct one above
How much faster is the rate of effusion of  than the rate of effusion of
than the rate of effusion of  ?
?
How much faster is the rate of effusion of 

By Graham's Law,  . The molar mass of
. The molar mass of  is 2 g/mol and the molar mass of
is 2 g/mol and the molar mass of  is 18 g/mol. Thus,
is 18 g/mol. Thus, 
By Graham's Law, 


Compare your answer with the correct one above
Suppose that an ideal gas at 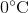 and 2.0 atm has a volume of 11.2 cubic meters. Which of the following expressions represents the volume of the gas, in cubic meters, if the temperature were increased by
and 2.0 atm has a volume of 11.2 cubic meters. Which of the following expressions represents the volume of the gas, in cubic meters, if the temperature were increased by  and the pressure decreased to 1.5 atm?
and the pressure decreased to 1.5 atm?
Suppose that an ideal gas at 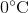

First of all, the unit degrees Celsius should be converted to Kelvins by adding 273. This means that the temperature increases from 273 K to 323 K.
Next, the combined gas law can be used.

Plugging in the known values gives

and solving for the volume gives

Rearranging shows this is clearly equivalent to the correct answer,  .
.
First of all, the unit degrees Celsius should be converted to Kelvins by adding 273. This means that the temperature increases from 273 K to 323 K.
Next, the combined gas law can be used.
Plugging in the known values gives
and solving for the volume gives
Rearranging shows this is clearly equivalent to the correct answer, 
Compare your answer with the correct one above


