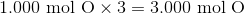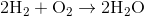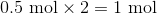SAT Subject Test in Chemistry - SAT Subject Test in Chemistry
Card 0 of 20
An atom of phosphorus has 15 protons, 16 neutrons, and 15 electrons. What is the atom's mass number?
An atom of phosphorus has 15 protons, 16 neutrons, and 15 electrons. What is the atom's mass number?
Protons and neutrons both have masses of approximately 1 amu, whereas the mass of an electron is negligible. Therefore, the mass number is equal to the number of protons and neutrons, which is 31.
Protons and neutrons both have masses of approximately 1 amu, whereas the mass of an electron is negligible. Therefore, the mass number is equal to the number of protons and neutrons, which is 31.
Compare your answer with the correct one above
How many nitrate ions are in one mole of  ?
?
How many nitrate ions are in one mole of 
Note that there are two nitrate ( ) ions for every formula unit of calcium nitrate. This means that in one mole of calcium nitrate, there are two moles of nitrate ions. To go from moles to ions, multiply 2 by Avogadro's number.
) ions for every formula unit of calcium nitrate. This means that in one mole of calcium nitrate, there are two moles of nitrate ions. To go from moles to ions, multiply 2 by Avogadro's number.

Note that there are two nitrate (
Compare your answer with the correct one above
How many hydrogen atoms are in one mole of  ?
?
How many hydrogen atoms are in one mole of 
In one molecule of  , there are 2 ammonium ions, each of which has 4 hydrogen atoms for a total of 8 hydrogen atoms. Thus, to find the number of hydrogen atoms in one mole of
, there are 2 ammonium ions, each of which has 4 hydrogen atoms for a total of 8 hydrogen atoms. Thus, to find the number of hydrogen atoms in one mole of  , one must multiply Avogadro's number by 8.
, one must multiply Avogadro's number by 8.

In one molecule of 

Compare your answer with the correct one above
Calculate the number of aluminum ions in  moles of
moles of  .
.
Calculate the number of aluminum ions in 

In order calculate the number of aluminum ions, we must first find the number of aluminum ions in the entire compound. In this case, there are two molecules of  for every molecule of
for every molecule of  . After understanding this, we can use Avogadro's constant to determine the number of atoms (more specifically ions) of aluminum. See equation below for specific calculations.
. After understanding this, we can use Avogadro's constant to determine the number of atoms (more specifically ions) of aluminum. See equation below for specific calculations.



In order calculate the number of aluminum ions, we must first find the number of aluminum ions in the entire compound. In this case, there are two molecules of 


Compare your answer with the correct one above
How many atoms of chloride are in 0.2550 g of aluminum chloride,  ?
?
How many atoms of chloride are in 0.2550 g of aluminum chloride, 
The formula for aluminum chloride is  . The molar mass of
. The molar mass of  is 26.98 g and the molar mass of
is 26.98 g and the molar mass of  is 35.45 g. There are three
is 35.45 g. There are three  atoms in
atoms in  . To calculate the molecular mass of
. To calculate the molecular mass of  , we need to find the sum of the mass of one aluminum atom and three chlorine atoms:
, we need to find the sum of the mass of one aluminum atom and three chlorine atoms:

The total molecular weight is 130.33 g. Starting with the grams of  , we convert to the amount of moles
, we convert to the amount of moles  using the molecular weight value we just calculated. Then we calculate the number of moles of chloride ion in
using the molecular weight value we just calculated. Then we calculate the number of moles of chloride ion in  . using the 3:1 ratio. Finally, we calculate the number of atoms by using Avogadro's number. See calculations below:
. using the 3:1 ratio. Finally, we calculate the number of atoms by using Avogadro's number. See calculations below:


The formula for aluminum chloride is 





The total molecular weight is 130.33 g. Starting with the grams of 


Compare your answer with the correct one above
When the equation for the reaction shown below is balanced with the lowest whole-number coefficients, what is the sum of the coefficients of the reactants?
___ 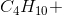 ___
___
 ___
___  ___
___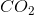
When the equation for the reaction shown below is balanced with the lowest whole-number coefficients, what is the sum of the coefficients of the reactants?
___ 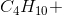



The coefficients of the equation, when balanced, should be 2, 13, 10, and 8. This problem wants to know the sum of the reactants, so only the coefficients on the left side of the reactants should be added. The sum of 2 and 13 is 15, so 15 is the correct answer.
The coefficients of the equation, when balanced, should be 2, 13, 10, and 8. This problem wants to know the sum of the reactants, so only the coefficients on the left side of the reactants should be added. The sum of 2 and 13 is 15, so 15 is the correct answer.
Compare your answer with the correct one above
What is the formula for the compound formed from calcium and sulfate ions?
What is the formula for the compound formed from calcium and sulfate ions?
In order to answer this question correctly, it is important to have knowledge of the formation of ionic bonds, polyatomic ions, and the periodic table. First, it is important to notice that calcium ions and sulfate ions will form an ionic bond. Knowing this, it can be understood that valance electrons will be exchanged between the ions and therefore, oppositely charged ions that are attracted to each other will be formed.
Calcium is in the second column of the periodic table, so it will provide two ions to the sulfate polyatomic ion. This will form a calcium cation ( ) and a sulfate polyatomic anion (
) and a sulfate polyatomic anion ( ).
).
One calcium ion provides two electrons, which are both accepted by a sulfate polyatomic ion, so the ratio of calcium ions used to sulfate polyatomic ions used is 1:1. This means that only one of each ion is needed to form the compound, making the formula  .
.
Initially it may be tempting to write the formula as  ; however, since both ions have a "2" subscript in this form, it is redundant to the formula in this way. Only one of each ion is needed to form the compound.
; however, since both ions have a "2" subscript in this form, it is redundant to the formula in this way. Only one of each ion is needed to form the compound.
In order to answer this question correctly, it is important to have knowledge of the formation of ionic bonds, polyatomic ions, and the periodic table. First, it is important to notice that calcium ions and sulfate ions will form an ionic bond. Knowing this, it can be understood that valance electrons will be exchanged between the ions and therefore, oppositely charged ions that are attracted to each other will be formed.
Calcium is in the second column of the periodic table, so it will provide two ions to the sulfate polyatomic ion. This will form a calcium cation (

One calcium ion provides two electrons, which are both accepted by a sulfate polyatomic ion, so the ratio of calcium ions used to sulfate polyatomic ions used is 1:1. This means that only one of each ion is needed to form the compound, making the formula 
Initially it may be tempting to write the formula as 
Compare your answer with the correct one above
30 mL of 1.0 M  solution is diluted with water to a volume of 3 L. What is the new concentration of the solution?
solution is diluted with water to a volume of 3 L. What is the new concentration of the solution?
30 mL of 1.0 M 
Recall that  Thus, the volume of the solution increased from 30 mL to 3000 mL, which is a factor of 100. Thus, the new concentration must be 100 times less than the original concentration, and
Thus, the volume of the solution increased from 30 mL to 3000 mL, which is a factor of 100. Thus, the new concentration must be 100 times less than the original concentration, and  .
.
Recall that 

Compare your answer with the correct one above
How much faster is the rate of effusion of  than the rate of effusion of
than the rate of effusion of  ?
?
How much faster is the rate of effusion of 

By Graham's Law,  . The molar mass of
. The molar mass of  is 2 g/mol and the molar mass of
is 2 g/mol and the molar mass of  is 18 g/mol. Thus,
is 18 g/mol. Thus, 
By Graham's Law, 


Compare your answer with the correct one above
Which of the following has the electron configuration of ![[Ne]3s^23p^6](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/1074018/gif.latex) ?
?
Which of the following has the electron configuration of ![[Ne]3s^23p^6](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/1074018/gif.latex)
The task is to find the atom or ion with its first three energy levels completely filled. Argon is not an answer choice, which means that it must be one of the ions with an atomic number close to argon's atomic number. Recalling that a positive charge means electrons were lost from the outermost shell and that a negative charge means electrons were gained in the outermost shell, the only answer that works is  .
.
The task is to find the atom or ion with its first three energy levels completely filled. Argon is not an answer choice, which means that it must be one of the ions with an atomic number close to argon's atomic number. Recalling that a positive charge means electrons were lost from the outermost shell and that a negative charge means electrons were gained in the outermost shell, the only answer that works is 
Compare your answer with the correct one above
Which pair of formulas represents the empirical formula and molecular formula for a certain compound?
Which pair of formulas represents the empirical formula and molecular formula for a certain compound?
To find an empirical formula, take a molecular formula and divide the subscript of each element by the greatest common factor of all the subscripts. In this case, the only pair that works is  , which can be verified by dividing the coefficients of the molecular formula by 6. Note that
, which can be verified by dividing the coefficients of the molecular formula by 6. Note that 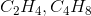 is not correct because neither of the formulas is an empirical formula; they are both possible molecular formulas for a compound with the empirical formula
is not correct because neither of the formulas is an empirical formula; they are both possible molecular formulas for a compound with the empirical formula  .
.
To find an empirical formula, take a molecular formula and divide the subscript of each element by the greatest common factor of all the subscripts. In this case, the only pair that works is 
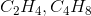

Compare your answer with the correct one above
Vanillin is composed of  carbon,
carbon,  oxygen, and
oxygen, and  hydrogen.
hydrogen.
What is the empirical formula of vanillin?
Refer to the following table for the atomic masses of the elements shown:

Vanillin is composed of 


What is the empirical formula of vanillin?
Refer to the following table for the atomic masses of the elements shown:
In order to calculate the empirical formula of vanillin from the given percent composition data, we can pretend we have a 100 g sample vanillin and from there estimate how many grams of each element make up the sample. For example, Vanillin is  carbon; therefore, the estimated sample would contain 66.3 g of carbon. After doing this for each of the elements vanillin contains, we can use the atomic mass of carbon to find how many moles of carbon make up the sample. We then do the same for each element that composes vanillin. See calculations below:
carbon; therefore, the estimated sample would contain 66.3 g of carbon. After doing this for each of the elements vanillin contains, we can use the atomic mass of carbon to find how many moles of carbon make up the sample. We then do the same for each element that composes vanillin. See calculations below:



After finding the number of moles of each element in the hypothetical 100 g sample, we look at the ratio between the moles of each element by dividing all the samples by smallest number of moles. After finding the ratios of the samples, we are able to use the empirical formula of the vanillin by rounding up to the nearest whole number for each sample. If the ratios are not close enough to round up (like in this example), we multiply the ratios all by the same number until each number is equal to a whole number nicely. See calculations below:



We need whole numbers, so multiply each of the resulting numbers by 3 to get rid of the fractions.


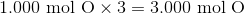
This gives us  as the final answer.
as the final answer.
In order to calculate the empirical formula of vanillin from the given percent composition data, we can pretend we have a 100 g sample vanillin and from there estimate how many grams of each element make up the sample. For example, Vanillin is 
After finding the number of moles of each element in the hypothetical 100 g sample, we look at the ratio between the moles of each element by dividing all the samples by smallest number of moles. After finding the ratios of the samples, we are able to use the empirical formula of the vanillin by rounding up to the nearest whole number for each sample. If the ratios are not close enough to round up (like in this example), we multiply the ratios all by the same number until each number is equal to a whole number nicely. See calculations below:
We need whole numbers, so multiply each of the resulting numbers by 3 to get rid of the fractions.
This gives us 
Compare your answer with the correct one above

Suppose that 1.5 moles of potassium reacts completely with an excess of chlorine gas. What is the enthalpy change of the reaction?
Suppose that 1.5 moles of potassium reacts completely with an excess of chlorine gas. What is the enthalpy change of the reaction?
First, note that heat is a product of the reaction. This means that the reaction releases heat and is exothermic, so the enthalpy change must be negative. Next, consider that 2 moles of sodium would react to produce 640 kJ. Thus, 1.5 moles of sodium would react to produce
 .
.
Hence, the enthalpy change is 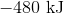 .
.
First, note that heat is a product of the reaction. This means that the reaction releases heat and is exothermic, so the enthalpy change must be negative. Next, consider that 2 moles of sodium would react to produce 640 kJ. Thus, 1.5 moles of sodium would react to produce

Hence, the enthalpy change is 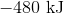
Compare your answer with the correct one above

What is an expression for the equilibrium constant for the above equation?
What is an expression for the equilibrium constant for the above equation?
The formula for the equilibrium constant is the product of the concentration of the products divided by the product of the concentration of the reactants. Since none of the reactants or products have coefficients, no exponents are needed in the expression. The phases do not affect the equilibrium constant in this example.
The formula for the equilibrium constant is the product of the concentration of the products divided by the product of the concentration of the reactants. Since none of the reactants or products have coefficients, no exponents are needed in the expression. The phases do not affect the equilibrium constant in this example.
Compare your answer with the correct one above
Suppose that an ideal gas at 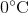 and 2.0 atm has a volume of 11.2 cubic meters. Which of the following expressions represents the volume of the gas, in cubic meters, if the temperature were increased by
and 2.0 atm has a volume of 11.2 cubic meters. Which of the following expressions represents the volume of the gas, in cubic meters, if the temperature were increased by  and the pressure decreased to 1.5 atm?
and the pressure decreased to 1.5 atm?
Suppose that an ideal gas at 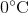

First of all, the unit degrees Celsius should be converted to Kelvins by adding 273. This means that the temperature increases from 273 K to 323 K.
Next, the combined gas law can be used.

Plugging in the known values gives

and solving for the volume gives

Rearranging shows this is clearly equivalent to the correct answer,  .
.
First of all, the unit degrees Celsius should be converted to Kelvins by adding 273. This means that the temperature increases from 273 K to 323 K.
Next, the combined gas law can be used.
Plugging in the known values gives
and solving for the volume gives
Rearranging shows this is clearly equivalent to the correct answer, 
Compare your answer with the correct one above
Which of the following does not exhibit hydrogen bonding as an intermolecular force?
Which of the following does not exhibit hydrogen bonding as an intermolecular force?
Hydrogen bonding occurs between hydrogen and a very electronegative atom, which is usually fluorine, oxygen, or nitrogen. Thus, hydrogen bonding does not occur in 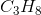 .
.
Hydrogen bonding occurs between hydrogen and a very electronegative atom, which is usually fluorine, oxygen, or nitrogen. Thus, hydrogen bonding does not occur in 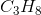
Compare your answer with the correct one above
Which of the following cannot act as a Bronsted-Lowry base (proton recipient) in aqueous solution?
Which of the following cannot act as a Bronsted-Lowry base (proton recipient) in aqueous solution?
 cannot receive another proton
cannot receive another proton  because then it would become
because then it would become  , which does not exist. All of the other answer choices are fine:
, which does not exist. All of the other answer choices are fine:

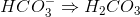





Compare your answer with the correct one above
In a solution, a weak electrolyte exists predominately as which of the following?
In a solution, a weak electrolyte exists predominately as which of the following?
An electrolyte is a substance that when dissolved in a solution breaks up into ions. More specifically, an electrolyte breaks up into cations (positively charged ions) and anions (negatively charged ions). While strong electrolytes break up 100% into anions and cations, weak electrolytes break apart significantly less. Less than 10% of a weak electrolyte ionizes at all in solution. That means the 90%–99% of the weak electrolyte's molecules do not ionize. This is because the intermolecular forces that form between the solution and the weak electrolyte's molecules are not stronger than the intramolecular forces holding the molecule together. While solutions containing weak electrolytes contain both ions formed from the weak electrolyte ions and molecules of the weak electrolyte, they contain mostly molecules of the weak electrolyte.
An electrolyte is a substance that when dissolved in a solution breaks up into ions. More specifically, an electrolyte breaks up into cations (positively charged ions) and anions (negatively charged ions). While strong electrolytes break up 100% into anions and cations, weak electrolytes break apart significantly less. Less than 10% of a weak electrolyte ionizes at all in solution. That means the 90%–99% of the weak electrolyte's molecules do not ionize. This is because the intermolecular forces that form between the solution and the weak electrolyte's molecules are not stronger than the intramolecular forces holding the molecule together. While solutions containing weak electrolytes contain both ions formed from the weak electrolyte ions and molecules of the weak electrolyte, they contain mostly molecules of the weak electrolyte.
Compare your answer with the correct one above
How many of the following compounds are ionic?





How many of the following compounds are ionic?
Ionic compounds are chemical compounds made of charged ions held together by forces called ionic bonding. Ionic bonds are formed by transfers of valance electrons, which create charged atoms (ions) which are attracted to each other because they have opposite charges. This is not be confused with covalent bonding, in which atoms form an attraction by sharing electrons.
 contains a positively charged
contains a positively charged  ion and negatively charged
ion and negatively charged 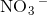 ion which form an ionic bond.
ion which form an ionic bond.  contains two positively charged
contains two positively charged  ions and a negatively charged
ions and a negatively charged  ion which form an ionic bond.
ion which form an ionic bond. 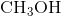 ,
,  , and
, and  are all examples of molecules that contain covalent bonds. None of them contains any charged ions. The answer is therefore "two,"referring to the
are all examples of molecules that contain covalent bonds. None of them contains any charged ions. The answer is therefore "two,"referring to the  and
and  molecules.
molecules.
Ionic compounds are chemical compounds made of charged ions held together by forces called ionic bonding. Ionic bonds are formed by transfers of valance electrons, which create charged atoms (ions) which are attracted to each other because they have opposite charges. This is not be confused with covalent bonding, in which atoms form an attraction by sharing electrons.


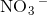



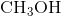




Compare your answer with the correct one above
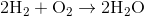
Suppose that 8 grams of of hydrogen gas and 16 grams of oxygen gas are ignited. What is the theoretical yield, in grams, of water? Assume hydrogen has an atomic mass of 1 and oxygen has an atomic mass of 16.
Suppose that 8 grams of of hydrogen gas and 16 grams of oxygen gas are ignited. What is the theoretical yield, in grams, of water? Assume hydrogen has an atomic mass of 1 and oxygen has an atomic mass of 16.
First, convert from grams to moles using the molar masses of the compounds. There are 4 mol of hydrogen gas and 0.5 mol of oxygen gas. Oxygen gas is clearly the limiting reagent (since there is more than twice as much hydrogen gas as oxygen gas), which means that the theoretical yield of water is
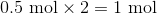
Water contains two hydrogen atoms and an oxygen atom, so it has a molar mass of

Hence, 1 mol of water would have a mass of 18 g.
First, convert from grams to moles using the molar masses of the compounds. There are 4 mol of hydrogen gas and 0.5 mol of oxygen gas. Oxygen gas is clearly the limiting reagent (since there is more than twice as much hydrogen gas as oxygen gas), which means that the theoretical yield of water is
Water contains two hydrogen atoms and an oxygen atom, so it has a molar mass of
Hence, 1 mol of water would have a mass of 18 g.
Compare your answer with the correct one above