Reaction Types - SAT Subject Test in Chemistry
Card 0 of 4
Which of the following cannot act as a Bronsted-Lowry base (proton recipient) in aqueous solution?
Which of the following cannot act as a Bronsted-Lowry base (proton recipient) in aqueous solution?
 cannot receive another proton
cannot receive another proton  because then it would become
because then it would become  , which does not exist. All of the other answer choices are fine:
, which does not exist. All of the other answer choices are fine:

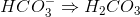





Compare your answer with the correct one above
What is the oxidation number of nitrogen in  ?
?
What is the oxidation number of nitrogen in 
First, note that the molecule does not have a charge, meaning that the oxidation numbers of each atom must add up to zero. Hydrogen has an oxidation number of  and oxygen has an oxidation number of
and oxygen has an oxidation number of  . Thus, if we call the oxidation number of nitrogen
. Thus, if we call the oxidation number of nitrogen  , we can get the equation
, we can get the equation  . Solving this gives
. Solving this gives  , so the oxidation number of nitrogen is
, so the oxidation number of nitrogen is  in this molecule.
in this molecule.
First, note that the molecule does not have a charge, meaning that the oxidation numbers of each atom must add up to zero. Hydrogen has an oxidation number of 





Compare your answer with the correct one above
The following is a modified true/false question. In it, you must decide if each individual statement is true (T) or false (F). If both are true, then you must also decide if the second statement is a correct explanation (CE) of the first statement.
I: An  ion would undergo reduction to form
ion would undergo reduction to form  metal
metal
BECAUSE
II: reduction is a loss of electrons
The following is a modified true/false question. In it, you must decide if each individual statement is true (T) or false (F). If both are true, then you must also decide if the second statement is a correct explanation (CE) of the first statement.
I: An 

BECAUSE
II: reduction is a loss of electrons
Statement II is false: reduction is a gain of electrons. Looking at statement I, the ion's charge would decrease by 3 in becoming a metal, which suggests that the ion gains 3 electrons. Thus, it is undergoing reduction.
Statement II is false: reduction is a gain of electrons. Looking at statement I, the ion's charge would decrease by 3 in becoming a metal, which suggests that the ion gains 3 electrons. Thus, it is undergoing reduction.
Compare your answer with the correct one above
A scientist makes a solution by adding 0.2 grams of  to enough water so that the resulting solution has a volume of 10 liters. What, approximately, is the pH of this solution?
to enough water so that the resulting solution has a volume of 10 liters. What, approximately, is the pH of this solution?
A scientist makes a solution by adding 0.2 grams of 
 has a molar mass of approximately 40 g/mol, meaning that there is 0.01 mol of it in the solution. Sodium hydroxide is a strong base and completely dissociates in water. Its concentration in the solution is
has a molar mass of approximately 40 g/mol, meaning that there is 0.01 mol of it in the solution. Sodium hydroxide is a strong base and completely dissociates in water. Its concentration in the solution is 
 . This means that the concentration of
. This means that the concentration of  ions is
ions is  and
and  . Thus, the pH of the solution is 11.
. Thus, the pH of the solution is 11.






Compare your answer with the correct one above