Acid-Base Reactions - SAT Subject Test in Chemistry
Card 0 of 2
Which of the following cannot act as a Bronsted-Lowry base (proton recipient) in aqueous solution?
Which of the following cannot act as a Bronsted-Lowry base (proton recipient) in aqueous solution?
 cannot receive another proton
cannot receive another proton  because then it would become
because then it would become  , which does not exist. All of the other answer choices are fine:
, which does not exist. All of the other answer choices are fine:

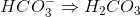





Compare your answer with the correct one above
A scientist makes a solution by adding 0.2 grams of  to enough water so that the resulting solution has a volume of 10 liters. What, approximately, is the pH of this solution?
to enough water so that the resulting solution has a volume of 10 liters. What, approximately, is the pH of this solution?
A scientist makes a solution by adding 0.2 grams of 
 has a molar mass of approximately 40 g/mol, meaning that there is 0.01 mol of it in the solution. Sodium hydroxide is a strong base and completely dissociates in water. Its concentration in the solution is
has a molar mass of approximately 40 g/mol, meaning that there is 0.01 mol of it in the solution. Sodium hydroxide is a strong base and completely dissociates in water. Its concentration in the solution is 
 . This means that the concentration of
. This means that the concentration of  ions is
ions is  and
and  . Thus, the pH of the solution is 11.
. Thus, the pH of the solution is 11.






Compare your answer with the correct one above