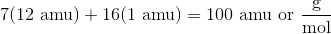Molar Mass - SAT Subject Test in Chemistry
Card 0 of 1
Question
What is the approximate percentage of carbon by mass in heptane, which has the molecular formula  ?
?
What is the approximate percentage of carbon by mass in heptane, which has the molecular formula 
Answer
First, calculate the molar mass of heptane, which is
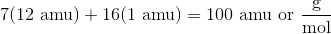
We can calculate the percentage of carbon by mass in heptane by creating a fraction of the mass of carbon over the mass of the entire molecule and converting it into a percentage:

For every 100 g of heptane, 84 g must come from carbon. This means that the percentage of carbon by mass is 
First, calculate the molar mass of heptane, which is
We can calculate the percentage of carbon by mass in heptane by creating a fraction of the mass of carbon over the mass of the entire molecule and converting it into a percentage:
For every 100 g of heptane, 84 g must come from carbon. This means that the percentage of carbon by mass is
Compare your answer with the correct one above
Tap the card to reveal the answer