Identifying and Defining Acids and Bases - SAT Subject Test in Chemistry
Card 0 of 1
Question
Which of the following cannot act as a Bronsted-Lowry base (proton recipient) in aqueous solution?
Which of the following cannot act as a Bronsted-Lowry base (proton recipient) in aqueous solution?
Answer
 cannot receive another proton
cannot receive another proton  because then it would become
because then it would become  , which does not exist. All of the other answer choices are fine:
, which does not exist. All of the other answer choices are fine:

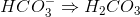





Compare your answer with the correct one above
Tap the card to reveal the answer