Gas Laws - SAT Subject Test in Chemistry
Card 0 of 1
Question
Suppose that an ideal gas at 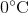 and 2.0 atm has a volume of 11.2 cubic meters. Which of the following expressions represents the volume of the gas, in cubic meters, if the temperature were increased by
and 2.0 atm has a volume of 11.2 cubic meters. Which of the following expressions represents the volume of the gas, in cubic meters, if the temperature were increased by  and the pressure decreased to 1.5 atm?
and the pressure decreased to 1.5 atm?
Suppose that an ideal gas at 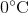

Answer
First of all, the unit degrees Celsius should be converted to Kelvins by adding 273. This means that the temperature increases from 273 K to 323 K.
Next, the combined gas law can be used.

Plugging in the known values gives

and solving for the volume gives

Rearranging shows this is clearly equivalent to the correct answer,  .
.
First of all, the unit degrees Celsius should be converted to Kelvins by adding 273. This means that the temperature increases from 273 K to 323 K.
Next, the combined gas law can be used.
Plugging in the known values gives
and solving for the volume gives
Rearranging shows this is clearly equivalent to the correct answer, 
Compare your answer with the correct one above
Tap the card to reveal the answer


