Molecules - SAT Subject Test in Chemistry
Card 0 of 2
Question
What is the molecular geometry of 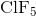 ?
?
What is the molecular geometry of 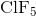
Answer
There are six electron domains in  .
.  has five bonding and one nonbonding pairs of electrons. The electron domain geometry of
has five bonding and one nonbonding pairs of electrons. The electron domain geometry of  is octahedral. When a molecule has octahedral electron domain geometry and has five bonding and one nonbonding pairs, the molecular geometry is square pyramidal.
is octahedral. When a molecule has octahedral electron domain geometry and has five bonding and one nonbonding pairs, the molecular geometry is square pyramidal.
There are six electron domains in 


Compare your answer with the correct one above
Question
Based on VSEPR theory and electron configurations, which of the following molecules is not matched with its correct geometry?
Based on VSEPR theory and electron configurations, which of the following molecules is not matched with its correct geometry?
Answer
 has three bonding pairs and one non-bonding pair, which is the geometry for trigonal pyramidal, not trigonal planar.
has three bonding pairs and one non-bonding pair, which is the geometry for trigonal pyramidal, not trigonal planar.

Compare your answer with the correct one above
Tap the card to reveal the answer