Gases
Practice Questions
SAT Subject Test in Chemistry › Gases
Questions
4
1
How much faster is the rate of effusion of 

2
How much faster is the rate of effusion of 

3
Suppose that an ideal gas at 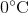

4
Suppose that an ideal gas at 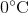

SAT Subject Test in Chemistry › Gases
How much faster is the rate of effusion of 

How much faster is the rate of effusion of 

Suppose that an ideal gas at 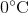

Suppose that an ideal gas at