Solutions and States of Matter
Practice Questions
SAT Subject Test in Chemistry › Solutions and States of Matter
30 mL of 1.0 M 
30 mL of 1.0 M 
30 mL of 1.0 M 
How much faster is the rate of effusion of 

How much faster is the rate of effusion of 

How much faster is the rate of effusion of 

Suppose that an ideal gas at 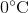

Suppose that an ideal gas at 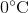

Suppose that an ideal gas at