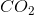SAT Subject Test in Chemistry
Practice Questions
SAT Subject Test in Chemistry › SAT Subject Test in Chemistry
How many nitrate ions are in one mole of 
How many nitrate ions are in one mole of 
How many nitrate ions are in one mole of 
How many hydrogen atoms are in one mole of 
How many hydrogen atoms are in one mole of 
How many hydrogen atoms are in one mole of 
When the equation for the reaction shown below is balanced with the lowest whole-number coefficients, what is the sum of the coefficients of the reactants?
___ 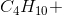



When the equation for the reaction shown below is balanced with the lowest whole-number coefficients, what is the sum of the coefficients of the reactants?
___ 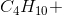



When the equation for the reaction shown below is balanced with the lowest whole-number coefficients, what is the sum of the coefficients of the reactants?
___ 



Octane, 
If 114 g of octane are burned in the complete combustion reaction shown below, how many grams of water will be produced?
___