Thermochemistry and Thermodynamics - Physical Chemistry
Card 0 of 20
The heat capacity of a bomb calorimeter assembly is  . What is the heat of combustion of
. What is the heat of combustion of  of sucrose in a bomb calorimeter that results in the temperature rising from
of sucrose in a bomb calorimeter that results in the temperature rising from  to
to  ?
?
The heat capacity of a bomb calorimeter assembly is 



A bomb calorimeter is a device used to measure the quantity of heat change for a process. The heat of a reaction which is denoted as  , is the negative of the thermal energy gained by the calorimeter:
, is the negative of the thermal energy gained by the calorimeter:

The heat capacity of a calorimeter is:

Plugging the values given into the equation gives:

Using the relation provided earlier:

Because we are dealing with 1.1 grams of sucrose, the heat of combustion of sucrose is:

A bomb calorimeter is a device used to measure the quantity of heat change for a process. The heat of a reaction which is denoted as 
The heat capacity of a calorimeter is:
Plugging the values given into the equation gives:
Using the relation provided earlier:
Because we are dealing with 1.1 grams of sucrose, the heat of combustion of sucrose is:
Compare your answer with the correct one above
The heat capacity of a bomb calorimeter assembly is  . What is the heat of combustion of
. What is the heat of combustion of  of caffeine in a bomb calorimeter that results in the temperature rising from
of caffeine in a bomb calorimeter that results in the temperature rising from  to
to  ?
?
The heat capacity of a bomb calorimeter assembly is 



A bomb calorimeter is a device used to measure the quantity of heat change for a process. The heat of a reaction which is denoted as q, is the negative of the thermal energy gained by the calorimeter:

The heat capacity of a calorimeter is:

Plugging the values given into the equation gives:

Using the relation provided earlier:

Because we are dealing with 1.65 grams of sucrose, the heat of combustion of sucrose is:

A bomb calorimeter is a device used to measure the quantity of heat change for a process. The heat of a reaction which is denoted as q, is the negative of the thermal energy gained by the calorimeter:
The heat capacity of a calorimeter is:
Plugging the values given into the equation gives:
Using the relation provided earlier:
Because we are dealing with 1.65 grams of sucrose, the heat of combustion of sucrose is:
Compare your answer with the correct one above
Which of the following are endothermic?
Which of the following are endothermic?
Endothermic processes involve a positive change in enthalpy. This means that the enthalpy of products is higher than the enthalpy of reactants and net heat energy is consumed. Phase changes that involve increasing the distance between particles (meaning conversion of solid to liquid (melting), liquid to gas (evaporation) and solid to gas (sublimation)) require an input of energy and are considered endothermic processes. On the other hand, phase changes that decrease the distance between particles (such as gas to liquid (condensation), liquid to solid (freezing), and gas to solid (deposition)) release energy and are considered exothermic processes.
Endothermic processes involve a positive change in enthalpy. This means that the enthalpy of products is higher than the enthalpy of reactants and net heat energy is consumed. Phase changes that involve increasing the distance between particles (meaning conversion of solid to liquid (melting), liquid to gas (evaporation) and solid to gas (sublimation)) require an input of energy and are considered endothermic processes. On the other hand, phase changes that decrease the distance between particles (such as gas to liquid (condensation), liquid to solid (freezing), and gas to solid (deposition)) release energy and are considered exothermic processes.
Compare your answer with the correct one above
Which of the following is true regarding an exothermic reaction?
I. Entropy always increases
II. It is always spontaneous
III. It can facilitate other energy-consuming reactions
Which of the following is true regarding an exothermic reaction?
I. Entropy always increases
II. It is always spontaneous
III. It can facilitate other energy-consuming reactions
Exothermic reactions involve release of heat energy. This means that the energy of the products is lower than the energy of the reactants. Entropy is the measure of disorder in a system. It does not depend on the enthalpy and can increase or decrease during an exothermic process. Spontaneity is determined by looking at the change in Gibbs free energy,  . Negative
. Negative  corresponds to a spontaneous process and positive
corresponds to a spontaneous process and positive  corresponds to a nonspontaneous process. While the equation for Gibbs free energy,
corresponds to a nonspontaneous process. While the equation for Gibbs free energy,  , involves change in enthalpy, the spontaneity also depends on temperature and change in entropy. Exothermic reactions release energy in the form of heat. This heat energy can be used to power other processes that require energy; therefore, exothermic reactions can facilitate active processes that consume energy.
, involves change in enthalpy, the spontaneity also depends on temperature and change in entropy. Exothermic reactions release energy in the form of heat. This heat energy can be used to power other processes that require energy; therefore, exothermic reactions can facilitate active processes that consume energy.
Exothermic reactions involve release of heat energy. This means that the energy of the products is lower than the energy of the reactants. Entropy is the measure of disorder in a system. It does not depend on the enthalpy and can increase or decrease during an exothermic process. Spontaneity is determined by looking at the change in Gibbs free energy, 



Compare your answer with the correct one above
In an __________ reaction, the products are more stable than the reactants; in an __________ reaction the reactants are more stable than the products.
In an __________ reaction, the products are more stable than the reactants; in an __________ reaction the reactants are more stable than the products.
Exergonic reactions release energy; therefore, the energy of products is lower than that of the reactants. Endergonic reactions consume energy; therefore, the energy of products is greater than that of the reactants. In other words, exergonic reactions are spontaneous, while endergonic reactions are nonspontaneous, and require the net input of energy to drive the reaction.
Exergonic reactions release energy; therefore, the energy of products is lower than that of the reactants. Endergonic reactions consume energy; therefore, the energy of products is greater than that of the reactants. In other words, exergonic reactions are spontaneous, while endergonic reactions are nonspontaneous, and require the net input of energy to drive the reaction.
Compare your answer with the correct one above
Consider the following spontaneous reaction:

What can you conclude about the enthalpy change in this reaction?
Consider the following spontaneous reaction:
What can you conclude about the enthalpy change in this reaction?
We need to use the following equation to answer this question:

Above,  is change in Gibbs free energy,
is change in Gibbs free energy,  is change in enthalpy,
is change in enthalpy,  is temperature, and
is temperature, and  is change in entropy. The question states that the reaction is spontaneous; therefore,
is change in entropy. The question states that the reaction is spontaneous; therefore,  is negative. We can also determine the
is negative. We can also determine the  for this reaction by looking at the phases of the products and reactants. Recall that entropy is a measure of disorder. When it comes to phases, gases have the highest entropy and solids have the lowest entropy. This is because in gases the molecules are spread out and have more room for disorder while solids are compressed and well packaged, decreasing the disorder of the atoms/molecules. Liquids are intermediate in entropy. In this reaction, we are creating a liquid from two molecules of gas; therefore, we are decreasing the entropy of the system (going to a more ordered, liquid state). The change in entropy for this reaction is negative.
for this reaction by looking at the phases of the products and reactants. Recall that entropy is a measure of disorder. When it comes to phases, gases have the highest entropy and solids have the lowest entropy. This is because in gases the molecules are spread out and have more room for disorder while solids are compressed and well packaged, decreasing the disorder of the atoms/molecules. Liquids are intermediate in entropy. In this reaction, we are creating a liquid from two molecules of gas; therefore, we are decreasing the entropy of the system (going to a more ordered, liquid state). The change in entropy for this reaction is negative.
Rearranging and solving the equation above for  gives us:
gives us:

Since both  and
and  are negative,
are negative,  will always be negative (regardless of temperature). This is an exothermic reaction.
will always be negative (regardless of temperature). This is an exothermic reaction.
We need to use the following equation to answer this question:
Above, 





Rearranging and solving the equation above for 
Since both 


Compare your answer with the correct one above
Which of the following is true regarding enthalpy and entropy?
Which of the following is true regarding enthalpy and entropy?
Enthalpy is the amount of internal energy contained in a compound whereas entropy is the amount of intrinsic disorder within the compound. Enthalpy is zero for elemental compounds such hydrogen gas and oxygen gas; therefore, enthalpy is nonzero for water (regardless of phase). Entropy, or the amount of disorder, is always highest for gases and lowest for solids. This is because gas molecules are widely spread out and, therefore, are more disordered than solids and liquids. Hydrogen gas will have a higher entropy than liquid water.
Enthalpy is the amount of internal energy contained in a compound whereas entropy is the amount of intrinsic disorder within the compound. Enthalpy is zero for elemental compounds such hydrogen gas and oxygen gas; therefore, enthalpy is nonzero for water (regardless of phase). Entropy, or the amount of disorder, is always highest for gases and lowest for solids. This is because gas molecules are widely spread out and, therefore, are more disordered than solids and liquids. Hydrogen gas will have a higher entropy than liquid water.
Compare your answer with the correct one above
According to the law of thermodynamics, which of the following statement(s) is/are true?
I. Enthalpy of a system is always increasing
II. Entropy of a system is always increasing
III. Absolute entropy can never be negative
According to the law of thermodynamics, which of the following statement(s) is/are true?
I. Enthalpy of a system is always increasing
II. Entropy of a system is always increasing
III. Absolute entropy can never be negative
The first law of thermodynamics states that the energy of the universe is always constant, which implies that energy cannot be created or destroyed. The energy lost by a system is gained by surroundings and vice versa; however, the total energy of the universe is always constant. The second law of thermodynamics states that the entropy, or the amount of disorder in the universe, is always increasing. This suggests that the universe is always going towards a more disordered state. Based on these two laws, we can determine that statement I and statement II are false. Note that these two statements are talking about the system, rather than the universe. The energy (in the form of enthalpy) and entropy can increase or decrease in a system. The surroundings will compensate accordingly to keep the energy of universe constant and increase the entropy. Absolute entropy of a system, surroundings or the universe can never be negative because it isn’t possible to have negative disorder (this is due to the definition of entropy; just remember that entropy can never be negative). Note that the change in entropy can, however, be negative.
The first law of thermodynamics states that the energy of the universe is always constant, which implies that energy cannot be created or destroyed. The energy lost by a system is gained by surroundings and vice versa; however, the total energy of the universe is always constant. The second law of thermodynamics states that the entropy, or the amount of disorder in the universe, is always increasing. This suggests that the universe is always going towards a more disordered state. Based on these two laws, we can determine that statement I and statement II are false. Note that these two statements are talking about the system, rather than the universe. The energy (in the form of enthalpy) and entropy can increase or decrease in a system. The surroundings will compensate accordingly to keep the energy of universe constant and increase the entropy. Absolute entropy of a system, surroundings or the universe can never be negative because it isn’t possible to have negative disorder (this is due to the definition of entropy; just remember that entropy can never be negative). Note that the change in entropy can, however, be negative.
Compare your answer with the correct one above
For Constant Temperature, Gibbs Free Energy is defined as:

Where ,  is the change in Gibbs Free Energy,
is the change in Gibbs Free Energy,  is the change in enthalpy,
is the change in enthalpy,  is temperature, and
is temperature, and  is the change in entropy.
is the change in entropy.
Which of the following scenarios is not possible?
For Constant Temperature, Gibbs Free Energy is defined as:
Where , 



Which of the following scenarios is not possible?
The following condition is not possible:

This is because if enthalpy is positive, and entropy is negative, the negative sign in front of the temperature term in the formula becomes positive. Addition of 2 positive numbers can not be negative. Plugging in arbitrary numbers into the other conditions can show they are all possible.
Take the following condition:

Then Gibbs free energy  can either be positive or negative, depending on the magnitude of enthalpy, entropy, and temperature. (If enthalpy is much larger than entropy and temperature, then the difference will be positive, but if entropy *
can either be positive or negative, depending on the magnitude of enthalpy, entropy, and temperature. (If enthalpy is much larger than entropy and temperature, then the difference will be positive, but if entropy *  is greater than the enthalpy, then the difference will be negative).
is greater than the enthalpy, then the difference will be negative).
The following condition is not possible:
This is because if enthalpy is positive, and entropy is negative, the negative sign in front of the temperature term in the formula becomes positive. Addition of 2 positive numbers can not be negative. Plugging in arbitrary numbers into the other conditions can show they are all possible.
Take the following condition:
Then Gibbs free energy 

Compare your answer with the correct one above
For constant temperature, Gibbs free energy is defined as:

Where ,  is the change in Gibbs free energy,
is the change in Gibbs free energy,  is the change in enthalpy,
is the change in enthalpy,  is temperature, and
is temperature, and 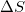 is the change in entropy.
is the change in entropy.
Given that a system is spontaneous, which of the following states are possible?
I. 
II. 
III. 
IV. 
For constant temperature, Gibbs free energy is defined as:
Where , 


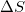
Given that a system is spontaneous, which of the following states are possible?
I.
II.
III.
IV.
Condition I is always true. Condition II is never true, as Gibbs free energy cannot be negative if enthalpy is positive and entropy is negative. Condition III may be true if temperature is very high (this is the scenario when the  term dominates the
term dominates the  term. Condition IV is not possible because
term. Condition IV is not possible because  and we were given a system with a Gibbs free Energy that is
and we were given a system with a Gibbs free Energy that is  (we were told the system was spontaneous).
(we were told the system was spontaneous).
Condition I is always true. Condition II is never true, as Gibbs free energy cannot be negative if enthalpy is positive and entropy is negative. Condition III may be true if temperature is very high (this is the scenario when the 



Compare your answer with the correct one above
The enthalpy of a reaction is  and the entropy of a reaction is
and the entropy of a reaction is  . Which of the following is the Gibbs free energy (in
. Which of the following is the Gibbs free energy (in  ) of this reaction?
) of this reaction?
The enthalpy of a reaction is 


Gibbs free energy of a system can be solved using the following equation.

where  is change in Gibbs free energy,
is change in Gibbs free energy,  is change in enthalpy,
is change in enthalpy,  is temperature in Kelvins and
is temperature in Kelvins and  is change in entropy. To solve for
is change in entropy. To solve for  we need all three of the variables. We are not given the temperature; therefore, we cannot solve for Gibbs free energy.
we need all three of the variables. We are not given the temperature; therefore, we cannot solve for Gibbs free energy.
Gibbs free energy of a system can be solved using the following equation.
where 




Compare your answer with the correct one above
In an exergonic reaction, products will have __________ Gibbs free energy and the reaction is __________.
In an exergonic reaction, products will have __________ Gibbs free energy and the reaction is __________.
Exergonic reaction suggests that the Gibbs free energy is negative. Since the change in Gibbs free energy is defined as Gibbs free energy of products - Gibbs free energy of reactants, a negative change in Gibbs free energy suggests that the products have a lower Gibbs free energy than reactants. A reaction is spontaneous if it has negative Gibbs free energy; therefore, exergonic reactions are always spontaneous. This is because the reaction is producing a more stable product (lower energy) from a less stable reactant (higher energy).
Exergonic reaction suggests that the Gibbs free energy is negative. Since the change in Gibbs free energy is defined as Gibbs free energy of products - Gibbs free energy of reactants, a negative change in Gibbs free energy suggests that the products have a lower Gibbs free energy than reactants. A reaction is spontaneous if it has negative Gibbs free energy; therefore, exergonic reactions are always spontaneous. This is because the reaction is producing a more stable product (lower energy) from a less stable reactant (higher energy).
Compare your answer with the correct one above
What is the specific heat capacity for a 50-gram sample of metal that increases in temperature by 10 degrees celsius when 2000 joules of energy is added?
What is the specific heat capacity for a 50-gram sample of metal that increases in temperature by 10 degrees celsius when 2000 joules of energy is added?
Use the equation:

We can calculate the specific heat capacity for the unknown metal. Since we know the added heat  , the mass of the sample
, the mass of the sample  , and the change in temperature
, and the change in temperature  , we can solve for
, we can solve for  .
.

Use the equation:
We can calculate the specific heat capacity for the unknown metal. Since we know the added heat 



Compare your answer with the correct one above
A  sample of an unknown metal at
sample of an unknown metal at  is immersed in water and is allowed to reach equilibrium. If final temperature of the system is
is immersed in water and is allowed to reach equilibrium. If final temperature of the system is 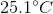 and
and  of energy are released, what is the identity of the metal?
of energy are released, what is the identity of the metal?

A 

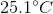


Recall that the formula for specific heat is  . Rearranging the equation for specific heat (c) yields
. Rearranging the equation for specific heat (c) yields 
Remark: keep in mind that the release of energy and the cooling of the metal will give negative values for both  and
and  .
.
Recall that the formula for specific heat is 
Remark: keep in mind that the release of energy and the cooling of the metal will give negative values for both 

Compare your answer with the correct one above
A chemical reaction is run in which  of work is done by the system and the internal energy changes by
of work is done by the system and the internal energy changes by  . What is the total amount of heat transferred?
. What is the total amount of heat transferred?
A chemical reaction is run in which 

The First Law of thermodynamics states that for a system that only exchanges energy by heat or work:

Work is done by the system, therefore



The First Law of thermodynamics states that for a system that only exchanges energy by heat or work:
Work is done by the system, therefore
Compare your answer with the correct one above
A chemical reaction is run in which  of heat is absorbed and the internal energy changes by
of heat is absorbed and the internal energy changes by  . What is the amount of work done?
. What is the amount of work done?
A chemical reaction is run in which 

The First Law of thermodynamics states that for a system that only exchanges energy by heat or work:

Heat is absorbed by the system, therefore

So 
 done by the system.
done by the system.
The First Law of thermodynamics states that for a system that only exchanges energy by heat or work:
Heat is absorbed by the system, therefore
So

Compare your answer with the correct one above
An automobile engine provides  of work to push the pistons and generates
of work to push the pistons and generates  of heat that must be carried away by the cooling system. What is the change in the internal energy of the engine?
of heat that must be carried away by the cooling system. What is the change in the internal energy of the engine?
An automobile engine provides 

The First Law of thermodynamics states that for a system that only exchanges energy by heat or work:

The heat is given off by the system, so  . Similarly, work is being done by the system, so
. Similarly, work is being done by the system, so 

The First Law of thermodynamics states that for a system that only exchanges energy by heat or work:
The heat is given off by the system, so 
Compare your answer with the correct one above
Which of the following is not true for an isothermal process?
Which of the following is not true for an isothermal process?
For an isothermal process there is no change in temperature, therefore, the temperature is a constant. An ideal gas has an internal energy,  , that is proportional to temperature, so if the temperature is does not change the internal energy does not change. Hence the name isothermal (iso means the same and thermal means temperature).
, that is proportional to temperature, so if the temperature is does not change the internal energy does not change. Hence the name isothermal (iso means the same and thermal means temperature).

If you rearrange the equation, you will find that:

A simple explanation of this is that all the heat applied to the system is used to do the work. A piston is a good example for this phenomenon. This happens when every bit of energy applied or removed from a piston as work is done so slowly that all the heat has enough time to conduct into or out of the system and maintain a constant temperature.
For an isothermal process there is no change in temperature, therefore, the temperature is a constant. An ideal gas has an internal energy, 
If you rearrange the equation, you will find that:
A simple explanation of this is that all the heat applied to the system is used to do the work. A piston is a good example for this phenomenon. This happens when every bit of energy applied or removed from a piston as work is done so slowly that all the heat has enough time to conduct into or out of the system and maintain a constant temperature.
Compare your answer with the correct one above
A sample of an ideal gas is initially at a volume of  . The gas expands to a volume of
. The gas expands to a volume of  when
when  of heat is applied to the system against a constant external pressure of
of heat is applied to the system against a constant external pressure of  . Calculate the change in internal energy for this gas.
. Calculate the change in internal energy for this gas.

A sample of an ideal gas is initially at a volume of 



The expression for the relationship between heat ( ) and work (
) and work ( ) is change in internal energy (
) is change in internal energy ( ):
):

The work ( ) done by a system is:
) done by a system is:

 with
with  and
and 
Plugging work ( ) into the internal energy equation gives:
) into the internal energy equation gives:

Plugging the values given into the internal energy equation gives:



The expression for the relationship between heat (


The work (


Plugging work (
Plugging the values given into the internal energy equation gives:
Compare your answer with the correct one above
At  , a
, a  sample of argon gas expands reversibly from a confined space of
sample of argon gas expands reversibly from a confined space of  to
to  . Calculate the work done.
. Calculate the work done.
At 



The process as described by the question is an isothermal process. An isothermal process has a constant temperature, therefore, there is no change in temperature. For an isothermal reversible process, the work done by the system is:

Plugging the values given into the equation gives:

The process as described by the question is an isothermal process. An isothermal process has a constant temperature, therefore, there is no change in temperature. For an isothermal reversible process, the work done by the system is:
Plugging the values given into the equation gives:
Compare your answer with the correct one above