Laws of Thermodynamics - Physical Chemistry
Card 0 of 1
A newly discovered element has been determined to have a standard entropy of 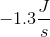 in its pure form. This value is highly unlikely because it violates the __________.
in its pure form. This value is highly unlikely because it violates the __________.
A newly discovered element has been determined to have a standard entropy of 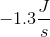
The Third Law of Thermodynamics states that a pure crystalline substance at absolute zero has an entropy of  . Mathematically, this formula is expressed as:
. Mathematically, this formula is expressed as:

where  is the Boltzmann constant
is the Boltzmann constant 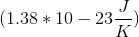 and W is the number of microstates of a gaseous atom in the system. Because microstates are used to describe this gaseous atom, there has to be at least one or more.
and W is the number of microstates of a gaseous atom in the system. Because microstates are used to describe this gaseous atom, there has to be at least one or more.
If only one microstate exists for the system, then the entropy is zero  , and can only increase from this point. It is therefore mathematically impossible for any element to have a negative standard entropy.
, and can only increase from this point. It is therefore mathematically impossible for any element to have a negative standard entropy.
Remark: keep in mind that entropy changes can be negative, but the entropy of pure compounds cannot be negative.
The Third Law of Thermodynamics states that a pure crystalline substance at absolute zero has an entropy of 
where 
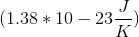
If only one microstate exists for the system, then the entropy is zero 
Remark: keep in mind that entropy changes can be negative, but the entropy of pure compounds cannot be negative.
Compare your answer with the correct one above