Freezing Point - Physical Chemistry
Card 0 of 1
Question
When 20 grams of a solute is added to a liter of water, the freezing point becomes  What is the molar mass of the solute?
What is the molar mass of the solute?
Assume that the solute does not make ions in solution.

When 20 grams of a solute is added to a liter of water, the freezing point becomes 
Assume that the solute does not make ions in solution.
Answer
We can use the freezing point depression equation in order to determine the molar mass of the unknown solute.

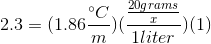

We can use the freezing point depression equation in order to determine the molar mass of the unknown solute.
Compare your answer with the correct one above
Tap the card to reveal the answer