Real and Ideal Gases
Practice Questions
Physical Chemistry › Real and Ideal Gases
Given the van der Waals Equation:
Which statement accurately identifies and explains the corrections that van der Waals made to the ideal gas law, where the first correction is the 

Given the ideal gas law:
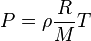
Where 




Based on the Ideal gas law, which of the following are true?
I. Pressure and volume are inversely proportional
II. Pressure and density are inversely proportional
III. Pressure and temperature are directly proportional
IV. Density and temperature are inversely proportional
V. R and M are inversely proportional
Under which conditions do gases deviate from ideality?
I. Low temperature
II. High temperature
III. Low pressure
IV. High pressure
V. Gases always behave ideally
