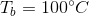Colligative Properties
Practice Questions
Physical Chemistry › Colligative Properties
Questions
6
1
Which of the following is not an intensive property?
2
Which of the following is not an intensive property?
3
What is the boiling point of a 100mL solution of water after the addition of 29g of sodium chloride?
4
What is the boiling point of a 100mL solution of water after the addition of 29g of sodium chloride?
5
When 20 grams of a solute is added to a liter of water, the freezing point becomes 
Assume that the solute does not make ions in solution.
6
When 20 grams of a solute is added to a liter of water, the freezing point becomes 
Assume that the solute does not make ions in solution.