Gibbs Free Energy
Practice Questions
Physical Chemistry › Gibbs Free Energy
In an exergonic reaction, products will have __________ Gibbs free energy and the reaction is __________.
For Constant Temperature, Gibbs Free Energy is defined as:
Where , 



Which of the following scenarios is not possible?
The enthalpy of a reaction is 


For constant temperature, Gibbs free energy is defined as:
Where , 


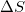
Given that a system is spontaneous, which of the following states are possible?
I.
II.
III.
IV.





