Carbonyl Reactions - Organic Chemistry
Card 0 of 11

Determine the major product of the given intramolecular aldol reaction.


Determine the major product of the given intramolecular aldol reaction.

Keep in mind the following principles: Cyclization is favored when a five/six-member ring may be formed. Addition at an aldehyde is favored relative to the same reaction at a ketone.
As a result, abstraction of a hydrogen bound to carbon 6 (an alpha-carbon) is favored since the resulting carbanion may attack the aldehyde (carbon 1) to form a six-member ring, resulting in compound II. Compound I results from abstracting a hydrogen from carbon 2, generating a carbanion which may then attack the ketone. Based on the latter of the above principles, this is a minor product.
Keep in mind the following principles: Cyclization is favored when a five/six-member ring may be formed. Addition at an aldehyde is favored relative to the same reaction at a ketone.
As a result, abstraction of a hydrogen bound to carbon 6 (an alpha-carbon) is favored since the resulting carbanion may attack the aldehyde (carbon 1) to form a six-member ring, resulting in compound II. Compound I results from abstracting a hydrogen from carbon 2, generating a carbanion which may then attack the ketone. Based on the latter of the above principles, this is a minor product.
Compare your answer with the correct one above
What is the final organic product of the reaction shown?


What is the final organic product of the reaction shown?


First step: Friedel-Crafts acylation of benzene
Second step: Formation of enolate
Third step: aldol addition (enolate attacks carbonyl carbon in benzaldehyde)
Fourth step: neutralization of anion and dehydration forming alkene
First step: Friedel-Crafts acylation of benzene
Second step: Formation of enolate
Third step: aldol addition (enolate attacks carbonyl carbon in benzaldehyde)
Fourth step: neutralization of anion and dehydration forming alkene
Compare your answer with the correct one above
What is the product of the following reaction?

What is the product of the following reaction?
The reaction uses a Grignard reagent's nucleophilic carbon to attack the carbon in carbon dioxide. Following treatment with water the resulting molecule (a carboxylate anion, of the form  ) the carboxylate oxygen will be protonated, and the result is a carboxylic acid. Thus, the resulting molecule is a benzene ring with a carboxyl group attached (this is also known as benzoic acid).
) the carboxylate oxygen will be protonated, and the result is a carboxylic acid. Thus, the resulting molecule is a benzene ring with a carboxyl group attached (this is also known as benzoic acid).
The reaction uses a Grignard reagent's nucleophilic carbon to attack the carbon in carbon dioxide. Following treatment with water the resulting molecule (a carboxylate anion, of the form 
Compare your answer with the correct one above

What is the product of this reaction?

What is the product of this reaction?
This is a classic esterification reaction. Esterfication occurs when a carboxylic acid and an alcohol are reacted together. Only one answer choice is an ester.
This is a classic esterification reaction. Esterfication occurs when a carboxylic acid and an alcohol are reacted together. Only one answer choice is an ester.
Compare your answer with the correct one above
Which of the following best summarizes the haloform reaction?
Which of the following best summarizes the haloform reaction?
The haloform reaction requires a carbonyl with a terminal alpha carbon. A hydrogen gets abstracted, and the enolate is formed. A halogen attacks the alpha carbon, and the ketone is reformed. This occurs three more times until the carbon has bonds to three halogens. Then the carbon leaves, forming a carbanion, and the base attacks the carbonyl carbon. An ester is formed.
The haloform reaction requires a carbonyl with a terminal alpha carbon. A hydrogen gets abstracted, and the enolate is formed. A halogen attacks the alpha carbon, and the ketone is reformed. This occurs three more times until the carbon has bonds to three halogens. Then the carbon leaves, forming a carbanion, and the base attacks the carbonyl carbon. An ester is formed.
Compare your answer with the correct one above
Which of the following best summarizes a Claisan condensation?
Which of the following best summarizes a Claisan condensation?
The most acidic hydrogen of the ester gets abstracted and the enolate form of the compound is attained. The electrons from the carbon-carbon double bond attack the carbonyl carbon of the other ester. Deesterfication occurs in this same step. The final product has one ketone and one ester.
The most acidic hydrogen of the ester gets abstracted and the enolate form of the compound is attained. The electrons from the carbon-carbon double bond attack the carbonyl carbon of the other ester. Deesterfication occurs in this same step. The final product has one ketone and one ester.
Compare your answer with the correct one above
Which of the following results in a single ketone product following acid catalyzed hydration?
Which of the following results in a single ketone product following acid catalyzed hydration?
During acid catalyzed hydration, a hydroxy group replaces one of the bonds in the triple bond and a double bond is formed. This is called an enol. The enol naturally turns into a ketone in a process called tautomerization. The hydroxy group can attach to either carbon across the double bond, and naming is done so that substituents have the lowest numbers. Only on 5-decyne will result in a single product, as no matter which carbon the hydroxy group bonds to, it is still on carbon 5. Thus, the only final product is 5-decone.
The other answer options will still react, but will form multiple products due to lack of symmetry.
During acid catalyzed hydration, a hydroxy group replaces one of the bonds in the triple bond and a double bond is formed. This is called an enol. The enol naturally turns into a ketone in a process called tautomerization. The hydroxy group can attach to either carbon across the double bond, and naming is done so that substituents have the lowest numbers. Only on 5-decyne will result in a single product, as no matter which carbon the hydroxy group bonds to, it is still on carbon 5. Thus, the only final product is 5-decone.
The other answer options will still react, but will form multiple products due to lack of symmetry.
Compare your answer with the correct one above
What is the product of the reaction shown?

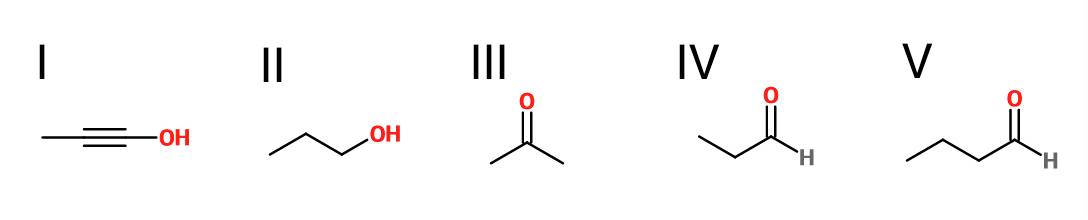
What is the product of the reaction shown?


First step: bromination across the double bond
Second step: double dehydrohalogenation and removal of terminal alkyne hydrogen
Third step: neutralization of the molecule
Fourth/fifth step: hydroboration/oxidation, followed by keto/enol tautomerization
First step: bromination across the double bond
Second step: double dehydrohalogenation and removal of terminal alkyne hydrogen
Third step: neutralization of the molecule
Fourth/fifth step: hydroboration/oxidation, followed by keto/enol tautomerization
Compare your answer with the correct one above
What is a product when propyl-butanoate undergoes saponification?
What is a product when propyl-butanoate undergoes saponification?
When naming esters, the root word in the name describes the carbon chain that carries the carbonyl. Saponification describes the breaking up of an ester. This yields an alcohol and a carboxylic acid. The chain with the carbonyl is butyl, so the carboxylic acid will be butanoic acid. That means the side with the alcohol will be propanol.
When naming esters, the root word in the name describes the carbon chain that carries the carbonyl. Saponification describes the breaking up of an ester. This yields an alcohol and a carboxylic acid. The chain with the carbonyl is butyl, so the carboxylic acid will be butanoic acid. That means the side with the alcohol will be propanol.
Compare your answer with the correct one above
All of the following are characteristics of a Wittig reaction except __________.
All of the following are characteristics of a Wittig reaction except __________.
The Wittig reaction involves the reaction of a phosphonium ylide (generated by treating a phosphonium salt with a strong base) with a ketone or aldehyde.

The reaction proceeds through a phosphaoxetane (4-membered ring containing both phosphorus and oxygen) intermediate to generate a new compound containing a carbon-carbon double bond, plus a phosphine oxide byproduct. It does not form trans double bonds exclusively; sometimes, a mixture of cis and trans isomers are obtained, and sometimes the cis isomer is the predominant product.
The Wittig reaction involves the reaction of a phosphonium ylide (generated by treating a phosphonium salt with a strong base) with a ketone or aldehyde.
The reaction proceeds through a phosphaoxetane (4-membered ring containing both phosphorus and oxygen) intermediate to generate a new compound containing a carbon-carbon double bond, plus a phosphine oxide byproduct. It does not form trans double bonds exclusively; sometimes, a mixture of cis and trans isomers are obtained, and sometimes the cis isomer is the predominant product.
Compare your answer with the correct one above
What is the final organic product of the reaction shown?


What is the final organic product of the reaction shown?


First step: esterification
Second step: reduction
Third step: neutralization
Fourth step: oxidation to aldehyde
Fifth step: alkene metathesis
First step: esterification
Second step: reduction
Third step: neutralization
Fourth step: oxidation to aldehyde
Fifth step: alkene metathesis
Compare your answer with the correct one above