Help with Intermolecular Forces
Practice Questions
Organic Chemistry › Help with Intermolecular Forces
Which of the following statements is true of alkynes?
Which of the following will have the highest vapor pressure?
Which of the following molecules has the highest boiling point?
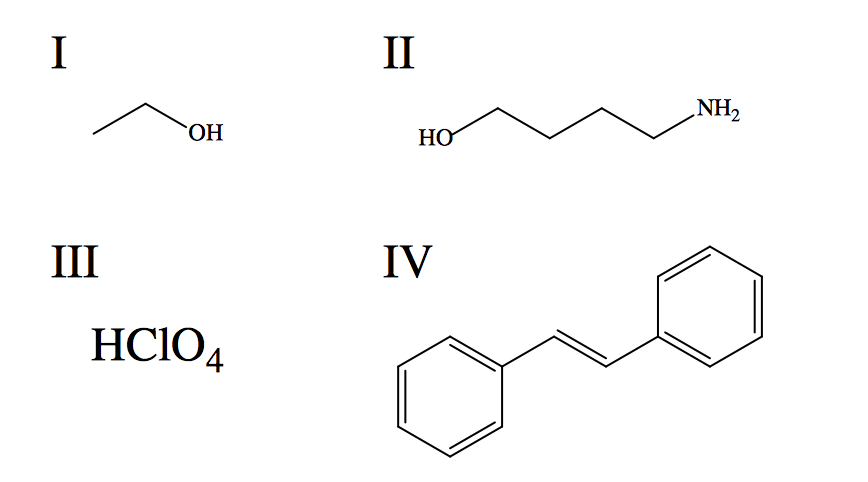
Rank the given species in terms of increasing aqueous solubility.
Which of the following element(s) is/are not involved in hydrogen bonds?
I. Nitrogen
II. Oxygen
III. Chlorine
Which of the following compounds are not able to form hydrogen bonds with water?
Which of these accurately describes hydrogen bonds?
Which of the following molecules would have the highest boiling point?
Of the following intermolecular forces, which force would typically provide a pure compound with the highest possible boiling point?
A researcher is trying to identify a molecule. He observes that there is a weak hydrophobic bond between adjacent molecules. He also notices a weak polar interaction between the molecules. Which of the following could be the identity of the molecule?