Substitution and Elimination Mechanisms - MCAT Biology
Card 0 of 16
Which of the following reactions is the nucleophile potassium tert-butoxide often used for?
Which of the following reactions is the nucleophile potassium tert-butoxide often used for?
Tert-butoxide is a large, sterically hindered, strong nucleophile that is often used in E2 reactions. Strong nucleophiles usually undergo the SN2 or E2 pathway, but tert-butoxide is much too large to undergo a substitution reaction.
Tert-butoxide is a large, sterically hindered, strong nucleophile that is often used in E2 reactions. Strong nucleophiles usually undergo the SN2 or E2 pathway, but tert-butoxide is much too large to undergo a substitution reaction.
Compare your answer with the correct one above
Which of the following factors do NOT favor an SN2 reaction of an alkyl halide?
Which of the following factors do NOT favor an SN2 reaction of an alkyl halide?
The way the question is phrased, three answer choices must favor an SN2 reaction, while the "correct" answer is a factor that does not favor, or disfavors an SN2 reaction.
SN2 reactions are bimolecular, and thus their rate of reaction depends on both the substrate and the nucleophile, forming a high energy transition state in which the nucleophile will displace the substate's leaving group at an angle of 180o. The more sterically hindered the compound is, the higher in energy the transition state will be, and the slower the rate of reaction will be. Consequently, SN2 reactions are favored when the leaving group (a halogen in this case) is on a primary carbon center. Additionally, because the reaction is bimolecular, step two of the reaction will NOT occur without a good nucleophile to displace the leaving group. Finally, all SN2 reactions are favored by polar aprotic solvents.
Because SN2 reactions proceed via a transition state, no carbocation intermediate is formed (that happens in SN1 reactions) and therefore the formation of any carbocation favors an SN1 reaction, not an SN2 reaction.
The way the question is phrased, three answer choices must favor an SN2 reaction, while the "correct" answer is a factor that does not favor, or disfavors an SN2 reaction.
SN2 reactions are bimolecular, and thus their rate of reaction depends on both the substrate and the nucleophile, forming a high energy transition state in which the nucleophile will displace the substate's leaving group at an angle of 180o. The more sterically hindered the compound is, the higher in energy the transition state will be, and the slower the rate of reaction will be. Consequently, SN2 reactions are favored when the leaving group (a halogen in this case) is on a primary carbon center. Additionally, because the reaction is bimolecular, step two of the reaction will NOT occur without a good nucleophile to displace the leaving group. Finally, all SN2 reactions are favored by polar aprotic solvents.
Because SN2 reactions proceed via a transition state, no carbocation intermediate is formed (that happens in SN1 reactions) and therefore the formation of any carbocation favors an SN1 reaction, not an SN2 reaction.
Compare your answer with the correct one above
When exposed to a good nucleophile, which molecule will most readily undergo an  reaction?
reaction?
When exposed to a good nucleophile, which molecule will most readily undergo an 
 reactions, also known as unimolecular nucleophilic substitution reactions, occur in two steps. Here, we are concerned with the first and second (rate-determining) steps, in which the leaving group breaks off of the molecule to form a carbocation. Alkanes that form the most stable carbocations are most likely to undergo
reactions, also known as unimolecular nucleophilic substitution reactions, occur in two steps. Here, we are concerned with the first and second (rate-determining) steps, in which the leaving group breaks off of the molecule to form a carbocation. Alkanes that form the most stable carbocations are most likely to undergo 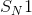 reactions. Tertiary carbocations are the most stable, followed by secondary. Primary and methyl carbocations are very unstable and unlikely to form at all. The tertiary alkane,
reactions. Tertiary carbocations are the most stable, followed by secondary. Primary and methyl carbocations are very unstable and unlikely to form at all. The tertiary alkane,  , will form a very stable tertiary carbocation compared to the other answer choices.
, will form a very stable tertiary carbocation compared to the other answer choices.


Compare your answer with the correct one above
Organic reactions can often be classified into two broad categories: substitution and elimination. Substitution reactions substitute one substituent for another. Elimination reactions typically form after the wholesale removal of a substituent, with no replacement. Below are examples of two types of reactions.
Reaction 1:

Reaction 2:

A scientist modifies reaction 1 by changing the reactant, removing a hydrogen from the central carbon and replacing it with a methyl group. The new reactant thus has two methyl groups and one hydrogen on the central carbon. What is true of reaction 1 following this modification? Assume the temperature remains constant and no catalyst is added.
Organic reactions can often be classified into two broad categories: substitution and elimination. Substitution reactions substitute one substituent for another. Elimination reactions typically form after the wholesale removal of a substituent, with no replacement. Below are examples of two types of reactions.
Reaction 1:

Reaction 2:

A scientist modifies reaction 1 by changing the reactant, removing a hydrogen from the central carbon and replacing it with a methyl group. The new reactant thus has two methyl groups and one hydrogen on the central carbon. What is true of reaction 1 following this modification? Assume the temperature remains constant and no catalyst is added.
Reaction 1 will experience greater steric hindrance with the addition of a methyl group, in place of a hydrogen, on the central carbon of the reactant. The result of this is increased activation energy, and a reduced rate of reaction in unchanging temperature and with no addition of a catalyst.
Reaction 1 will experience greater steric hindrance with the addition of a methyl group, in place of a hydrogen, on the central carbon of the reactant. The result of this is increased activation energy, and a reduced rate of reaction in unchanging temperature and with no addition of a catalyst.
Compare your answer with the correct one above
Organic reactions can often be classified into two broad categories: substitution and elimination. Substitution reactions substitute one substituent for another. Elimination reactions typically form after the wholesale removal of a substituent, with no replacement. Below are examples of two types of reactions.
Reaction 1:

Reaction 2:

Which of the following describe the intermediate in reaction 1?
I. It is planar
II. It is uncharged (neutral)
III. It is a carbocation
IV. Reaction 1 does not involve an intermediate
Organic reactions can often be classified into two broad categories: substitution and elimination. Substitution reactions substitute one substituent for another. Elimination reactions typically form after the wholesale removal of a substituent, with no replacement. Below are examples of two types of reactions.
Reaction 1:

Reaction 2:

Which of the following describe the intermediate in reaction 1?
I. It is planar
II. It is uncharged (neutral)
III. It is a carbocation
IV. Reaction 1 does not involve an intermediate
Intermediates are relatively stable, while transition states are unstable and transient. The transition state (not the intermediate) of reaction 1 is a planar uncharged structure; however, only relatively stable species such as carbocations are considered intermediates. Reaction 1 does not have an intermediate, and is an example of an SN2 reaction; only SN1 reactions use a carbocation intermediate.
Intermediates are relatively stable, while transition states are unstable and transient. The transition state (not the intermediate) of reaction 1 is a planar uncharged structure; however, only relatively stable species such as carbocations are considered intermediates. Reaction 1 does not have an intermediate, and is an example of an SN2 reaction; only SN1 reactions use a carbocation intermediate.
Compare your answer with the correct one above
Organic reactions can often be classified into two broad categories: substitution and elimination. Substitution reactions substitute one substituent for another. Elimination reactions typically form after the wholesale removal of a substituent, with no replacement. Below are examples of two types of reactions.
Reaction 1:

Reaction 2:

In reaction 2, which of the following describe the rate limiting step?
I. It involves the formation of carbocation
II. It is favored by the presence of substituents on the central carbon
III. It involves a transition state, but no intermediate
Organic reactions can often be classified into two broad categories: substitution and elimination. Substitution reactions substitute one substituent for another. Elimination reactions typically form after the wholesale removal of a substituent, with no replacement. Below are examples of two types of reactions.
Reaction 1:

Reaction 2:

In reaction 2, which of the following describe the rate limiting step?
I. It involves the formation of carbocation
II. It is favored by the presence of substituents on the central carbon
III. It involves a transition state, but no intermediate
Reaction 2 represents an E1 reaction. The rate limiting step of reaction 2 involves the formation of a carbocation, whose stability is favored by the presence of substituents on the carbon involved. Carbocations are considered intermediates due to their relative stability compared to transition states.
Reaction 2 represents an E1 reaction. The rate limiting step of reaction 2 involves the formation of a carbocation, whose stability is favored by the presence of substituents on the carbon involved. Carbocations are considered intermediates due to their relative stability compared to transition states.
Compare your answer with the correct one above
Organic reactions can often be classified into two broad categories: substitution and elimination. Substitution reactions substitute one substituent for another. Elimination reactions typically form after the wholesale removal of a substituent, with no replacement. Below are examples of two types of reactions.
Reaction 1:

Reaction 2:

In the rate limiting step of reaction 2, which of the following describe the intermediate chemical species?
I. It has sp2 hybridization
II. It is trigonal planar
III. It exhibits bond rigidity, limiting rotation
Organic reactions can often be classified into two broad categories: substitution and elimination. Substitution reactions substitute one substituent for another. Elimination reactions typically form after the wholesale removal of a substituent, with no replacement. Below are examples of two types of reactions.
Reaction 1:

Reaction 2:

In the rate limiting step of reaction 2, which of the following describe the intermediate chemical species?
I. It has sp2 hybridization
II. It is trigonal planar
III. It exhibits bond rigidity, limiting rotation
Reaction 2 is an E1 reaction, in which the rate limiting step is the formation of the carbocation intermediate. The carbocation intermediate has three single bonds and a positive charge on the central carbon; thus, it has sp2 hybridization, a planar structure, and free rotation about its single bonds. Bond rigidity is only observed with the presence of pi bonds.
Reaction 2 is an E1 reaction, in which the rate limiting step is the formation of the carbocation intermediate. The carbocation intermediate has three single bonds and a positive charge on the central carbon; thus, it has sp2 hybridization, a planar structure, and free rotation about its single bonds. Bond rigidity is only observed with the presence of pi bonds.
Compare your answer with the correct one above
Organic reactions can often be classified into two broad categories: substitution and elimination. Substitution reactions substitute one substituent for another. Elimination reactions typically form after the wholesale removal of a substituent, with no replacement. Below are examples of two types of reactions.
Reaction 1:

Reaction 2:

If reaction 1 were modified and a water molecule was used in place of the hydroxide ion, which of the following would likely be true?
Organic reactions can often be classified into two broad categories: substitution and elimination. Substitution reactions substitute one substituent for another. Elimination reactions typically form after the wholesale removal of a substituent, with no replacement. Below are examples of two types of reactions.
Reaction 1:

Reaction 2:

If reaction 1 were modified and a water molecule was used in place of the hydroxide ion, which of the following would likely be true?
Reaction 1 represents an SN2 reaction. Such reactions depend, in part on the presence of strong nucleophiles, such as the hydroxide ion. Water can be a nucleophile as well, but it is weaker. Using water in place of hydroxide would cause reaction 1 to proceed far more slowly.
Reaction 1 represents an SN2 reaction. Such reactions depend, in part on the presence of strong nucleophiles, such as the hydroxide ion. Water can be a nucleophile as well, but it is weaker. Using water in place of hydroxide would cause reaction 1 to proceed far more slowly.
Compare your answer with the correct one above

The above image undergoes an E1 elimination reaction in a lab. The researchers note that the major product formed was the "Zaitsev" product. Which of the following compounds did the observers see most abundantly when the reaction was complete?

The above image undergoes an E1 elimination reaction in a lab. The researchers note that the major product formed was the "Zaitsev" product. Which of the following compounds did the observers see most abundantly when the reaction was complete?
The Zaitsev product is the most stable alkene that can be formed. This is the major product formed in E1 elimination reactions, because the carbocation can undergo hydride shifts to stabilize the positive charge. The most stable alkene is the most substituted alkene, and thus the correct answer.

The Zaitsev product is the most stable alkene that can be formed. This is the major product formed in E1 elimination reactions, because the carbocation can undergo hydride shifts to stabilize the positive charge. The most stable alkene is the most substituted alkene, and thus the correct answer.

Compare your answer with the correct one above
Organic reactions can often be classified into two broad categories: substitution and elimination. Substitution reactions substitute one substituent for another. Elimination reactions typically form after the wholesale removal of a substituent, with no replacement. Below are examples of two types of reactions.
Reaction 1:

Reaction 2:

A scientist is studying the rate of reaction 1. He wants to double the rate of the reaction, but is unsure how to increase concentrations of the reactants. Which of the following is true?
Organic reactions can often be classified into two broad categories: substitution and elimination. Substitution reactions substitute one substituent for another. Elimination reactions typically form after the wholesale removal of a substituent, with no replacement. Below are examples of two types of reactions.
Reaction 1:

Reaction 2:

A scientist is studying the rate of reaction 1. He wants to double the rate of the reaction, but is unsure how to increase concentrations of the reactants. Which of the following is true?
Reaction 1 represents an SN2 reaction. The rate limiting step involves both reactants coming together to form a transition state. The rate of this reaction depends on the concentration of both the organic molecule and the nucleophile.
In contrast, reaction 2 is an E1 reaction, in which the rate limiting step is the removal of the leaving group to form a carbocation. In E1 and SN1 reactions, adjusting the concentration of the halide only is enough to affect the rate.
Reaction 1 represents an SN2 reaction. The rate limiting step involves both reactants coming together to form a transition state. The rate of this reaction depends on the concentration of both the organic molecule and the nucleophile.
In contrast, reaction 2 is an E1 reaction, in which the rate limiting step is the removal of the leaving group to form a carbocation. In E1 and SN1 reactions, adjusting the concentration of the halide only is enough to affect the rate.
Compare your answer with the correct one above
Organic reactions can often be classified into two broad categories: substitution and elimination. Substitution reactions substitute one substituent for another. Elimination reactions typically form after the wholesale removal of a substituent, with no replacement. Below are examples of two types of reactions.
Reaction 1:

Reaction 2:

Investigating reaction 2, you find that the reaction is initiated when a carbocation forms. Which of the following is likely true?
I. Concentration of the halide is the main determinant of reaction rate
II. The carbocation forms when the hydroxide removes the chlorine atom
III. The carbocation is planar
Organic reactions can often be classified into two broad categories: substitution and elimination. Substitution reactions substitute one substituent for another. Elimination reactions typically form after the wholesale removal of a substituent, with no replacement. Below are examples of two types of reactions.
Reaction 1:

Reaction 2:

Investigating reaction 2, you find that the reaction is initiated when a carbocation forms. Which of the following is likely true?
I. Concentration of the halide is the main determinant of reaction rate
II. The carbocation forms when the hydroxide removes the chlorine atom
III. The carbocation is planar
The carbocation forms spontaneously with the loss of the chlorine atom. This is the rate determining step, thus, the concentration of the halide is the most important determinant of reaction rate. Carbocations form spontaneously in these reactions, and do not use the strong base to remove the halogen.
The carbocation forms spontaneously with the loss of the chlorine atom. This is the rate determining step, thus, the concentration of the halide is the most important determinant of reaction rate. Carbocations form spontaneously in these reactions, and do not use the strong base to remove the halogen.
Compare your answer with the correct one above
Organic reactions can often be classified into two broad categories: substitution and elimination. Substitution reactions substitute one substituent for another. Elimination reactions typically form after the wholesale removal of a substituent, with no replacement. Below are examples of two types of reactions.
Reaction 1:

Reaction 2:

Using the product of reaction 2, a scientist adds bromine gas to the reaction chamber. After the bromine and the alkene react, he finds that his product consists entirely of single bonds, with two bromine atoms on the carbon chain. What kind of reaction most likely took place?
Organic reactions can often be classified into two broad categories: substitution and elimination. Substitution reactions substitute one substituent for another. Elimination reactions typically form after the wholesale removal of a substituent, with no replacement. Below are examples of two types of reactions.
Reaction 1:

Reaction 2:

Using the product of reaction 2, a scientist adds bromine gas to the reaction chamber. After the bromine and the alkene react, he finds that his product consists entirely of single bonds, with two bromine atoms on the carbon chain. What kind of reaction most likely took place?
The addition of bromine gas ( ) to the reaction vessel would likely result in the addition of one half of the diatomic bromine to each carbon, eliminating the double bond and resulting in an alkyl halide chain.
) to the reaction vessel would likely result in the addition of one half of the diatomic bromine to each carbon, eliminating the double bond and resulting in an alkyl halide chain.
Halogenation reactions refer to reactions between a halogen and an alkane, while addition reactions occur between a halogen and an alkene (such as the product in reaction 2).
The addition of bromine gas (
Halogenation reactions refer to reactions between a halogen and an alkane, while addition reactions occur between a halogen and an alkene (such as the product in reaction 2).
Compare your answer with the correct one above
Organic reactions can often be classified into two broad categories: substitution and elimination. Substitution reactions substitute one substituent for another. Elimination reactions typically form after the wholesale removal of a substituent, with no replacement. Below are examples of two types of reactions.
Reaction 1:

Reaction 2:

The reaction depicted in reaction 1 takes place in solution with a solvent. What type of solvent is most likely to be preferred for the reaction to occur as written?
Organic reactions can often be classified into two broad categories: substitution and elimination. Substitution reactions substitute one substituent for another. Elimination reactions typically form after the wholesale removal of a substituent, with no replacement. Below are examples of two types of reactions.
Reaction 1:

Reaction 2:

The reaction depicted in reaction 1 takes place in solution with a solvent. What type of solvent is most likely to be preferred for the reaction to occur as written?
Reaction 1 is an SN2 reaction. This type of substitution reaction prefers a polar, aprotic solvent. The polarity helps to solvate the nucleophile. Aprotic solvents help mediate the transition state and increase reaction rate.
Reaction 1 is an SN2 reaction. This type of substitution reaction prefers a polar, aprotic solvent. The polarity helps to solvate the nucleophile. Aprotic solvents help mediate the transition state and increase reaction rate.
Compare your answer with the correct one above
Organic reactions can often be classified into two broad categories: substitution and elimination. Substitution reactions substitute one substituent for another. Elimination reactions typically form after the wholesale removal of a substituent, with no replacement. Below are examples of two types of reactions.
Reaction 1:

Reaction 2:

In reaction 1, a scientist is trying to modify the reaction by using a weaker nucleophile. Which of the following is a weaker nucleophile than what is used above (hydroxide ions)?
Organic reactions can often be classified into two broad categories: substitution and elimination. Substitution reactions substitute one substituent for another. Elimination reactions typically form after the wholesale removal of a substituent, with no replacement. Below are examples of two types of reactions.
Reaction 1:

Reaction 2:

In reaction 1, a scientist is trying to modify the reaction by using a weaker nucleophile. Which of the following is a weaker nucleophile than what is used above (hydroxide ions)?
Nucleophilicity increases to the left on the periodic table. Nucleophilicity will also generally increase with charge. The only equally charged ion in the answers that is present to the right of oxygen on the periodic table is the fluoride ion.
Nucleophilicity increases to the left on the periodic table. Nucleophilicity will also generally increase with charge. The only equally charged ion in the answers that is present to the right of oxygen on the periodic table is the fluoride ion.
Compare your answer with the correct one above

Students in an organic chemistry lab perform two E2 elimination reactions, using compounds 1 and 2. The students observe that while compound 2 undergoes elimination with reagent X (not shown), compound 1 is unreactive. What is the best explanation for this discrepancy?

Students in an organic chemistry lab perform two E2 elimination reactions, using compounds 1 and 2. The students observe that while compound 2 undergoes elimination with reagent X (not shown), compound 1 is unreactive. What is the best explanation for this discrepancy?
This question tests your understanding on E2 elimination reactions. Because E2 reactions have no carbocation intermediates, the reaction proceeds through a transition state. The bromine atom (Br) must be displaced by the bond between an adjacent carbon and hydrogen, and that hydrogen must be anti-periplanar to the bromine. Compound 2, unlike compound 1 has one hydrogen that is anti-periplanar to the bromine, and thus undergoes an E2 elimination.
If compound 2 reacts, it is unlikely the reason that compound 1 would not react is because of steric hindrance. Additionally, aside from stereochemistry, there is nothing different enough about the two compounds that would suggest any reagent would be more favorable for one over the other.
This question tests your understanding on E2 elimination reactions. Because E2 reactions have no carbocation intermediates, the reaction proceeds through a transition state. The bromine atom (Br) must be displaced by the bond between an adjacent carbon and hydrogen, and that hydrogen must be anti-periplanar to the bromine. Compound 2, unlike compound 1 has one hydrogen that is anti-periplanar to the bromine, and thus undergoes an E2 elimination.
If compound 2 reacts, it is unlikely the reason that compound 1 would not react is because of steric hindrance. Additionally, aside from stereochemistry, there is nothing different enough about the two compounds that would suggest any reagent would be more favorable for one over the other.
Compare your answer with the correct one above
Which of the following compounds could NEVER undergo an E2 reaction when treated with potassium tert-butoxide?
Which of the following compounds could NEVER undergo an E2 reaction when treated with potassium tert-butoxide?
For an E2 reaction to occur, there must be a hydrogen on the carbon adjacent to the carbon with the leaving group. Benzyl bromide contains no hydrogens on the carbon next to the carbon with the bromide, and would therefore undergo only a substitution reaction.
For an E2 reaction to occur, there must be a hydrogen on the carbon adjacent to the carbon with the leaving group. Benzyl bromide contains no hydrogens on the carbon next to the carbon with the bromide, and would therefore undergo only a substitution reaction.
Compare your answer with the correct one above