Reaction Mechanisms - MCAT Biology
Card 0 of 20
The transformation of compound B to compound C below is known as what type of reaction?

The transformation of compound B to compound C below is known as what type of reaction?

The conversion of compound B to compound Cresults in the elimination of water, which, by definition, is a dehydration reaction. The hydroxyl group on compound B is protonated by the sulfuric acid, generating an  leaving group and allowing the formation of the alkene product in compound C.
leaving group and allowing the formation of the alkene product in compound C.
Hydroboration is the oxidation of an alkene with a borohydride (usually sodium borohydride) reagent, to produce an alkane. Hydration involves the use of a water reactant, usually producing an alcohol product. Hydrogenation is the oxidation of alkene double bonds with the use of a palladium interface to produce an alkane product. Decarboxylation results in product carbon dioxide from a carboxylic acid or carbonic anhydrase reactant.
The conversion of compound B to compound Cresults in the elimination of water, which, by definition, is a dehydration reaction. The hydroxyl group on compound B is protonated by the sulfuric acid, generating an 
Hydroboration is the oxidation of an alkene with a borohydride (usually sodium borohydride) reagent, to produce an alkane. Hydration involves the use of a water reactant, usually producing an alcohol product. Hydrogenation is the oxidation of alkene double bonds with the use of a palladium interface to produce an alkane product. Decarboxylation results in product carbon dioxide from a carboxylic acid or carbonic anhydrase reactant.
Compare your answer with the correct one above
Which alcohol will react most rapidly via an SN1 mechanism?
Which alcohol will react most rapidly via an SN1 mechanism?
Tertiary alcohols react most rapidly via SN1 mechanisms because they form stable tertiary carbocations. Primary and secondary alcohols typically react most rapidly via SN2 mechanisms.
Of the available options,  is the only one that contains a tertiary alcohol.
is the only one that contains a tertiary alcohol.
Tertiary alcohols react most rapidly via SN1 mechanisms because they form stable tertiary carbocations. Primary and secondary alcohols typically react most rapidly via SN2 mechanisms.
Of the available options, 
Compare your answer with the correct one above
What type of enzymatic inhibitor binds to an allosteric location on the enzyme with equal affinity for the bound and unbound substrate states?
What type of enzymatic inhibitor binds to an allosteric location on the enzyme with equal affinity for the bound and unbound substrate states?
Noncompetitive inhibitors are a specific type of mixed inhibitor that binds to both the free enzyme and the enzyme-substrate complex with equal affinities, resulting in the same binding affinity (Km value) but a decrease in the maximum rate of reaction (Vmax value).
Noncompetitive inhibitors are a specific type of mixed inhibitor that binds to both the free enzyme and the enzyme-substrate complex with equal affinities, resulting in the same binding affinity (Km value) but a decrease in the maximum rate of reaction (Vmax value).
Compare your answer with the correct one above
The enzyme glucose-6-phosphatase plays the most important role in which tissue/organ?
The enzyme glucose-6-phosphatase plays the most important role in which tissue/organ?
Glucose-6-phosphatase is responsible for removing the phosphate group from glucose-6-phosphate. The result is free glucose, which can be released into the blood. This process takes place at the end of either glycogenolysis or gluconeogenesis, both processes that are most prominent in the liver due to its large stores of glycogen.
Glucose-6-phosphatase is responsible for removing the phosphate group from glucose-6-phosphate. The result is free glucose, which can be released into the blood. This process takes place at the end of either glycogenolysis or gluconeogenesis, both processes that are most prominent in the liver due to its large stores of glycogen.
Compare your answer with the correct one above
Which of the following statements is false regarding enzyme function?
Which of the following statements is false regarding enzyme function?
Enzymes function as a biological catalysts by lowering reactions' activation energies. They are not used up in the reaction mechanism, nor do they affect the thermodynamics of the reaction. They only affect the reaction kinetics.
Enzymes function as a biological catalysts by lowering reactions' activation energies. They are not used up in the reaction mechanism, nor do they affect the thermodynamics of the reaction. They only affect the reaction kinetics.
Compare your answer with the correct one above
The cellular membrane is a very important structure. The lipid bilayer is both hydrophilic and hydrophobic. The hydrophilic layer faces the extracellular fluid and the cytosol of the cell. The hydrophobic portion of the lipid bilayer stays in between the hydrophobic regions like a sandwich. This bilayer separation allows for communication, protection, and homeostasis.
One of the most utilized signaling transduction pathways is the G protein-coupled receptor pathway. The hydrophobic and hydrophilic properties of the cellular membrane allows for the peptide and other hydrophilic hormones to bind to the receptor on the cellular surface but to not enter the cell. This regulation allows for activation despite the hormone’s short half-life. On the other hand, hydrophobic hormones must have longer half-lives to allow for these ligands to cross the lipid bilayer, travel through the cell’s cytosol and eventually reach the nucleus.
Cholesterol allows the lipid bilayer to maintain its fluidity despite the fluctuation in the body’s temperature due to events such as increasing metabolism. Cholesterol binds to the hydrophobic tails of the lipid bilayer. When the temperature is low, the cholesterol molecules prevent the hydrophobic tails from compacting and solidifying. When the temperature is high, the hydrophobic tails will be excited and will move excessively. This excess movement will bring instability to the bilayer. Cholesterol will prevent excessive movement.
Epinephrine binds to its receptor on the surface of the cell. Molecule A does bind to the same receptor but is found to bind a different part of the receptor molecule than does epinephrine, causing the receptor to undergo a confirmation change and no longer fits with its associated ligand. What type of regulation is this?
The cellular membrane is a very important structure. The lipid bilayer is both hydrophilic and hydrophobic. The hydrophilic layer faces the extracellular fluid and the cytosol of the cell. The hydrophobic portion of the lipid bilayer stays in between the hydrophobic regions like a sandwich. This bilayer separation allows for communication, protection, and homeostasis.
One of the most utilized signaling transduction pathways is the G protein-coupled receptor pathway. The hydrophobic and hydrophilic properties of the cellular membrane allows for the peptide and other hydrophilic hormones to bind to the receptor on the cellular surface but to not enter the cell. This regulation allows for activation despite the hormone’s short half-life. On the other hand, hydrophobic hormones must have longer half-lives to allow for these ligands to cross the lipid bilayer, travel through the cell’s cytosol and eventually reach the nucleus.
Cholesterol allows the lipid bilayer to maintain its fluidity despite the fluctuation in the body’s temperature due to events such as increasing metabolism. Cholesterol binds to the hydrophobic tails of the lipid bilayer. When the temperature is low, the cholesterol molecules prevent the hydrophobic tails from compacting and solidifying. When the temperature is high, the hydrophobic tails will be excited and will move excessively. This excess movement will bring instability to the bilayer. Cholesterol will prevent excessive movement.
Epinephrine binds to its receptor on the surface of the cell. Molecule A does bind to the same receptor but is found to bind a different part of the receptor molecule than does epinephrine, causing the receptor to undergo a confirmation change and no longer fits with its associated ligand. What type of regulation is this?
According to the question, molecule A acts on a different site to inhibit epinephrine's receptor. This is an example of a noncompetitive inhibitor.
According to the question, molecule A acts on a different site to inhibit epinephrine's receptor. This is an example of a noncompetitive inhibitor.
Compare your answer with the correct one above
Which of the following compounds can be oxidized to form a ketone?
Which of the following compounds can be oxidized to form a ketone?
An alcohol can be oxidized and form a ketone only if the alcohol is a secondary alcohol. Tertiary alcohols cannot be oxidized and primary alcohols are oxidized to form aldehydes and carboxylic acids.
3-pentanol is a secondary alcohol, while 2-methyl-2-butanol is a tertiary alcohol. Methanol and propyl alcohol are both primary alcohols.
An alcohol can be oxidized and form a ketone only if the alcohol is a secondary alcohol. Tertiary alcohols cannot be oxidized and primary alcohols are oxidized to form aldehydes and carboxylic acids.
3-pentanol is a secondary alcohol, while 2-methyl-2-butanol is a tertiary alcohol. Methanol and propyl alcohol are both primary alcohols.
Compare your answer with the correct one above
What reagent would be best suited to accomplish the transformation shown below?

What reagent would be best suited to accomplish the transformation shown below?

Reduction of an ester to an alcohol requires a strong reducing agent, such as  .
.
 is not a strong enough reducing agent for this reaction. Hydrogenation and reaction with zinc would reduce the nitro group to an amino group instead of affecting the carboxylic acid.
is not a strong enough reducing agent for this reaction. Hydrogenation and reaction with zinc would reduce the nitro group to an amino group instead of affecting the carboxylic acid. 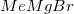 is a Grignard reagent, and would not function as a reducing agent.
is a Grignard reagent, and would not function as a reducing agent.
Reduction of an ester to an alcohol requires a strong reducing agent, such as 

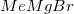
Compare your answer with the correct one above
Which of the following reactions is the nucleophile potassium tert-butoxide often used for?
Which of the following reactions is the nucleophile potassium tert-butoxide often used for?
Tert-butoxide is a large, sterically hindered, strong nucleophile that is often used in E2 reactions. Strong nucleophiles usually undergo the SN2 or E2 pathway, but tert-butoxide is much too large to undergo a substitution reaction.
Tert-butoxide is a large, sterically hindered, strong nucleophile that is often used in E2 reactions. Strong nucleophiles usually undergo the SN2 or E2 pathway, but tert-butoxide is much too large to undergo a substitution reaction.
Compare your answer with the correct one above
Which of the following factors do NOT favor an SN2 reaction of an alkyl halide?
Which of the following factors do NOT favor an SN2 reaction of an alkyl halide?
The way the question is phrased, three answer choices must favor an SN2 reaction, while the "correct" answer is a factor that does not favor, or disfavors an SN2 reaction.
SN2 reactions are bimolecular, and thus their rate of reaction depends on both the substrate and the nucleophile, forming a high energy transition state in which the nucleophile will displace the substate's leaving group at an angle of 180o. The more sterically hindered the compound is, the higher in energy the transition state will be, and the slower the rate of reaction will be. Consequently, SN2 reactions are favored when the leaving group (a halogen in this case) is on a primary carbon center. Additionally, because the reaction is bimolecular, step two of the reaction will NOT occur without a good nucleophile to displace the leaving group. Finally, all SN2 reactions are favored by polar aprotic solvents.
Because SN2 reactions proceed via a transition state, no carbocation intermediate is formed (that happens in SN1 reactions) and therefore the formation of any carbocation favors an SN1 reaction, not an SN2 reaction.
The way the question is phrased, three answer choices must favor an SN2 reaction, while the "correct" answer is a factor that does not favor, or disfavors an SN2 reaction.
SN2 reactions are bimolecular, and thus their rate of reaction depends on both the substrate and the nucleophile, forming a high energy transition state in which the nucleophile will displace the substate's leaving group at an angle of 180o. The more sterically hindered the compound is, the higher in energy the transition state will be, and the slower the rate of reaction will be. Consequently, SN2 reactions are favored when the leaving group (a halogen in this case) is on a primary carbon center. Additionally, because the reaction is bimolecular, step two of the reaction will NOT occur without a good nucleophile to displace the leaving group. Finally, all SN2 reactions are favored by polar aprotic solvents.
Because SN2 reactions proceed via a transition state, no carbocation intermediate is formed (that happens in SN1 reactions) and therefore the formation of any carbocation favors an SN1 reaction, not an SN2 reaction.
Compare your answer with the correct one above
When exposed to a good nucleophile, which molecule will most readily undergo an  reaction?
reaction?
When exposed to a good nucleophile, which molecule will most readily undergo an 
 reactions, also known as unimolecular nucleophilic substitution reactions, occur in two steps. Here, we are concerned with the first and second (rate-determining) steps, in which the leaving group breaks off of the molecule to form a carbocation. Alkanes that form the most stable carbocations are most likely to undergo
reactions, also known as unimolecular nucleophilic substitution reactions, occur in two steps. Here, we are concerned with the first and second (rate-determining) steps, in which the leaving group breaks off of the molecule to form a carbocation. Alkanes that form the most stable carbocations are most likely to undergo 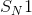 reactions. Tertiary carbocations are the most stable, followed by secondary. Primary and methyl carbocations are very unstable and unlikely to form at all. The tertiary alkane,
reactions. Tertiary carbocations are the most stable, followed by secondary. Primary and methyl carbocations are very unstable and unlikely to form at all. The tertiary alkane,  , will form a very stable tertiary carbocation compared to the other answer choices.
, will form a very stable tertiary carbocation compared to the other answer choices.

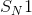

Compare your answer with the correct one above
Organic reactions can often be classified into two broad categories: substitution and elimination. Substitution reactions substitute one substituent for another. Elimination reactions typically form after the wholesale removal of a substituent, with no replacement. Below are examples of two types of reactions.
Reaction 1:

Reaction 2:

A scientist modifies reaction 1 by changing the reactant, removing a hydrogen from the central carbon and replacing it with a methyl group. The new reactant thus has two methyl groups and one hydrogen on the central carbon. What is true of reaction 1 following this modification? Assume the temperature remains constant and no catalyst is added.
Organic reactions can often be classified into two broad categories: substitution and elimination. Substitution reactions substitute one substituent for another. Elimination reactions typically form after the wholesale removal of a substituent, with no replacement. Below are examples of two types of reactions.
Reaction 1:

Reaction 2:

A scientist modifies reaction 1 by changing the reactant, removing a hydrogen from the central carbon and replacing it with a methyl group. The new reactant thus has two methyl groups and one hydrogen on the central carbon. What is true of reaction 1 following this modification? Assume the temperature remains constant and no catalyst is added.
Reaction 1 will experience greater steric hindrance with the addition of a methyl group, in place of a hydrogen, on the central carbon of the reactant. The result of this is increased activation energy, and a reduced rate of reaction in unchanging temperature and with no addition of a catalyst.
Reaction 1 will experience greater steric hindrance with the addition of a methyl group, in place of a hydrogen, on the central carbon of the reactant. The result of this is increased activation energy, and a reduced rate of reaction in unchanging temperature and with no addition of a catalyst.
Compare your answer with the correct one above
Organic reactions can often be classified into two broad categories: substitution and elimination. Substitution reactions substitute one substituent for another. Elimination reactions typically form after the wholesale removal of a substituent, with no replacement. Below are examples of two types of reactions.
Reaction 1:

Reaction 2:

Which of the following describe the intermediate in reaction 1?
I. It is planar
II. It is uncharged (neutral)
III. It is a carbocation
IV. Reaction 1 does not involve an intermediate
Organic reactions can often be classified into two broad categories: substitution and elimination. Substitution reactions substitute one substituent for another. Elimination reactions typically form after the wholesale removal of a substituent, with no replacement. Below are examples of two types of reactions.
Reaction 1:

Reaction 2:

Which of the following describe the intermediate in reaction 1?
I. It is planar
II. It is uncharged (neutral)
III. It is a carbocation
IV. Reaction 1 does not involve an intermediate
Intermediates are relatively stable, while transition states are unstable and transient. The transition state (not the intermediate) of reaction 1 is a planar uncharged structure; however, only relatively stable species such as carbocations are considered intermediates. Reaction 1 does not have an intermediate, and is an example of an SN2 reaction; only SN1 reactions use a carbocation intermediate.
Intermediates are relatively stable, while transition states are unstable and transient. The transition state (not the intermediate) of reaction 1 is a planar uncharged structure; however, only relatively stable species such as carbocations are considered intermediates. Reaction 1 does not have an intermediate, and is an example of an SN2 reaction; only SN1 reactions use a carbocation intermediate.
Compare your answer with the correct one above
Organic reactions can often be classified into two broad categories: substitution and elimination. Substitution reactions substitute one substituent for another. Elimination reactions typically form after the wholesale removal of a substituent, with no replacement. Below are examples of two types of reactions.
Reaction 1:

Reaction 2:

In reaction 2, which of the following describe the rate limiting step?
I. It involves the formation of carbocation
II. It is favored by the presence of substituents on the central carbon
III. It involves a transition state, but no intermediate
Organic reactions can often be classified into two broad categories: substitution and elimination. Substitution reactions substitute one substituent for another. Elimination reactions typically form after the wholesale removal of a substituent, with no replacement. Below are examples of two types of reactions.
Reaction 1:

Reaction 2:

In reaction 2, which of the following describe the rate limiting step?
I. It involves the formation of carbocation
II. It is favored by the presence of substituents on the central carbon
III. It involves a transition state, but no intermediate
Reaction 2 represents an E1 reaction. The rate limiting step of reaction 2 involves the formation of a carbocation, whose stability is favored by the presence of substituents on the carbon involved. Carbocations are considered intermediates due to their relative stability compared to transition states.
Reaction 2 represents an E1 reaction. The rate limiting step of reaction 2 involves the formation of a carbocation, whose stability is favored by the presence of substituents on the carbon involved. Carbocations are considered intermediates due to their relative stability compared to transition states.
Compare your answer with the correct one above
Organic reactions can often be classified into two broad categories: substitution and elimination. Substitution reactions substitute one substituent for another. Elimination reactions typically form after the wholesale removal of a substituent, with no replacement. Below are examples of two types of reactions.
Reaction 1:

Reaction 2:

In the rate limiting step of reaction 2, which of the following describe the intermediate chemical species?
I. It has sp2 hybridization
II. It is trigonal planar
III. It exhibits bond rigidity, limiting rotation
Organic reactions can often be classified into two broad categories: substitution and elimination. Substitution reactions substitute one substituent for another. Elimination reactions typically form after the wholesale removal of a substituent, with no replacement. Below are examples of two types of reactions.
Reaction 1:

Reaction 2:

In the rate limiting step of reaction 2, which of the following describe the intermediate chemical species?
I. It has sp2 hybridization
II. It is trigonal planar
III. It exhibits bond rigidity, limiting rotation
Reaction 2 is an E1 reaction, in which the rate limiting step is the formation of the carbocation intermediate. The carbocation intermediate has three single bonds and a positive charge on the central carbon; thus, it has sp2 hybridization, a planar structure, and free rotation about its single bonds. Bond rigidity is only observed with the presence of pi bonds.
Reaction 2 is an E1 reaction, in which the rate limiting step is the formation of the carbocation intermediate. The carbocation intermediate has three single bonds and a positive charge on the central carbon; thus, it has sp2 hybridization, a planar structure, and free rotation about its single bonds. Bond rigidity is only observed with the presence of pi bonds.
Compare your answer with the correct one above
Organic reactions can often be classified into two broad categories: substitution and elimination. Substitution reactions substitute one substituent for another. Elimination reactions typically form after the wholesale removal of a substituent, with no replacement. Below are examples of two types of reactions.
Reaction 1:

Reaction 2:

If reaction 1 were modified and a water molecule was used in place of the hydroxide ion, which of the following would likely be true?
Organic reactions can often be classified into two broad categories: substitution and elimination. Substitution reactions substitute one substituent for another. Elimination reactions typically form after the wholesale removal of a substituent, with no replacement. Below are examples of two types of reactions.
Reaction 1:

Reaction 2:

If reaction 1 were modified and a water molecule was used in place of the hydroxide ion, which of the following would likely be true?
Reaction 1 represents an SN2 reaction. Such reactions depend, in part on the presence of strong nucleophiles, such as the hydroxide ion. Water can be a nucleophile as well, but it is weaker. Using water in place of hydroxide would cause reaction 1 to proceed far more slowly.
Reaction 1 represents an SN2 reaction. Such reactions depend, in part on the presence of strong nucleophiles, such as the hydroxide ion. Water can be a nucleophile as well, but it is weaker. Using water in place of hydroxide would cause reaction 1 to proceed far more slowly.
Compare your answer with the correct one above

The above image undergoes an E1 elimination reaction in a lab. The researchers note that the major product formed was the "Zaitsev" product. Which of the following compounds did the observers see most abundantly when the reaction was complete?

The above image undergoes an E1 elimination reaction in a lab. The researchers note that the major product formed was the "Zaitsev" product. Which of the following compounds did the observers see most abundantly when the reaction was complete?
The Zaitsev product is the most stable alkene that can be formed. This is the major product formed in E1 elimination reactions, because the carbocation can undergo hydride shifts to stabilize the positive charge. The most stable alkene is the most substituted alkene, and thus the correct answer.

The Zaitsev product is the most stable alkene that can be formed. This is the major product formed in E1 elimination reactions, because the carbocation can undergo hydride shifts to stabilize the positive charge. The most stable alkene is the most substituted alkene, and thus the correct answer.

Compare your answer with the correct one above
Organic reactions can often be classified into two broad categories: substitution and elimination. Substitution reactions substitute one substituent for another. Elimination reactions typically form after the wholesale removal of a substituent, with no replacement. Below are examples of two types of reactions.
Reaction 1:

Reaction 2:

A scientist is studying the rate of reaction 1. He wants to double the rate of the reaction, but is unsure how to increase concentrations of the reactants. Which of the following is true?
Organic reactions can often be classified into two broad categories: substitution and elimination. Substitution reactions substitute one substituent for another. Elimination reactions typically form after the wholesale removal of a substituent, with no replacement. Below are examples of two types of reactions.
Reaction 1:

Reaction 2:

A scientist is studying the rate of reaction 1. He wants to double the rate of the reaction, but is unsure how to increase concentrations of the reactants. Which of the following is true?
Reaction 1 represents an SN2 reaction. The rate limiting step involves both reactants coming together to form a transition state. The rate of this reaction depends on the concentration of both the organic molecule and the nucleophile.
In contrast, reaction 2 is an E1 reaction, in which the rate limiting step is the removal of the leaving group to form a carbocation. In E1 and SN1 reactions, adjusting the concentration of the halide only is enough to affect the rate.
Reaction 1 represents an SN2 reaction. The rate limiting step involves both reactants coming together to form a transition state. The rate of this reaction depends on the concentration of both the organic molecule and the nucleophile.
In contrast, reaction 2 is an E1 reaction, in which the rate limiting step is the removal of the leaving group to form a carbocation. In E1 and SN1 reactions, adjusting the concentration of the halide only is enough to affect the rate.
Compare your answer with the correct one above
Organic reactions can often be classified into two broad categories: substitution and elimination. Substitution reactions substitute one substituent for another. Elimination reactions typically form after the wholesale removal of a substituent, with no replacement. Below are examples of two types of reactions.
Reaction 1:

Reaction 2:

Investigating reaction 2, you find that the reaction is initiated when a carbocation forms. Which of the following is likely true?
I. Concentration of the halide is the main determinant of reaction rate
II. The carbocation forms when the hydroxide removes the chlorine atom
III. The carbocation is planar
Organic reactions can often be classified into two broad categories: substitution and elimination. Substitution reactions substitute one substituent for another. Elimination reactions typically form after the wholesale removal of a substituent, with no replacement. Below are examples of two types of reactions.
Reaction 1:

Reaction 2:

Investigating reaction 2, you find that the reaction is initiated when a carbocation forms. Which of the following is likely true?
I. Concentration of the halide is the main determinant of reaction rate
II. The carbocation forms when the hydroxide removes the chlorine atom
III. The carbocation is planar
The carbocation forms spontaneously with the loss of the chlorine atom. This is the rate determining step, thus, the concentration of the halide is the most important determinant of reaction rate. Carbocations form spontaneously in these reactions, and do not use the strong base to remove the halogen.
The carbocation forms spontaneously with the loss of the chlorine atom. This is the rate determining step, thus, the concentration of the halide is the most important determinant of reaction rate. Carbocations form spontaneously in these reactions, and do not use the strong base to remove the halogen.
Compare your answer with the correct one above
Organic reactions can often be classified into two broad categories: substitution and elimination. Substitution reactions substitute one substituent for another. Elimination reactions typically form after the wholesale removal of a substituent, with no replacement. Below are examples of two types of reactions.
Reaction 1:

Reaction 2:

Using the product of reaction 2, a scientist adds bromine gas to the reaction chamber. After the bromine and the alkene react, he finds that his product consists entirely of single bonds, with two bromine atoms on the carbon chain. What kind of reaction most likely took place?
Organic reactions can often be classified into two broad categories: substitution and elimination. Substitution reactions substitute one substituent for another. Elimination reactions typically form after the wholesale removal of a substituent, with no replacement. Below are examples of two types of reactions.
Reaction 1:

Reaction 2:

Using the product of reaction 2, a scientist adds bromine gas to the reaction chamber. After the bromine and the alkene react, he finds that his product consists entirely of single bonds, with two bromine atoms on the carbon chain. What kind of reaction most likely took place?
The addition of bromine gas ( ) to the reaction vessel would likely result in the addition of one half of the diatomic bromine to each carbon, eliminating the double bond and resulting in an alkyl halide chain.
) to the reaction vessel would likely result in the addition of one half of the diatomic bromine to each carbon, eliminating the double bond and resulting in an alkyl halide chain.
Halogenation reactions refer to reactions between a halogen and an alkane, while addition reactions occur between a halogen and an alkene (such as the product in reaction 2).
The addition of bromine gas (
Halogenation reactions refer to reactions between a halogen and an alkane, while addition reactions occur between a halogen and an alkene (such as the product in reaction 2).
Compare your answer with the correct one above