Protein Structure - MCAT Biology
Card 0 of 20
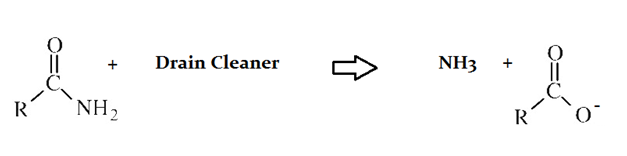
Drain cleaners are a common household staple, used to open clogged drains in bathtubs and sinks. Human hair is a common culprit that clogs pipes, and hair is made predominately of protein. Drain cleaners are effective at breaking down proteins that have accumulated in plumbing. Drain cleaners can be either acidic or basic, and are also effective at breaking down fats that have accumulated with proteins.
A typical reaction—reaction 1—which would be expected for a drain cleaner on contact with human hair, would be as follows in an aqueous solution:
Another reaction that may occur, reaction 2, would take place as follows in an aqueous solution:
In the proteins depicted in both reactions in the preceeding passage, which portions of the molecule are shown?
I. Amino terminus
II. Carboxy terminius
III. Side chain
Drain cleaners are a common household staple, used to open clogged drains in bathtubs and sinks. Human hair is a common culprit that clogs pipes, and hair is made predominately of protein. Drain cleaners are effective at breaking down proteins that have accumulated in plumbing. Drain cleaners can be either acidic or basic, and are also effective at breaking down fats that have accumulated with proteins.
A typical reaction—reaction 1—which would be expected for a drain cleaner on contact with human hair, would be as follows in an aqueous solution:
Another reaction that may occur, reaction 2, would take place as follows in an aqueous solution:
In the proteins depicted in both reactions in the preceeding passage, which portions of the molecule are shown?
I. Amino terminus
II. Carboxy terminius
III. Side chain
The protein that is reacting with the drain cleaner in both instances shows the NH2 end, or amino terminus. The side chain and carboxy terminus are not shown.
The protein that is reacting with the drain cleaner in both instances shows the NH2 end, or amino terminus. The side chain and carboxy terminus are not shown.
Compare your answer with the correct one above
Which of the following statements is NOT true regarding the comparison of the alpha-helix structure to the beta-sheet structure in proteins?
Which of the following statements is NOT true regarding the comparison of the alpha-helix structure to the beta-sheet structure in proteins?
Alpha-helices and beta-sheets are secondary structure motifs that occur when sequences of amino acids are linked by hydrogen bonds. These secondary structures are an integral part of globular proteins, such as hemoglobin. Alpha-helices resemble a coiled spring, with hydrogen bonding occurring in an intra-chain arrangement between carbonyl oxygens and amide hydrogens that is parallel to the central axis. Beta sheets, on the other hand, may have either inter- or intra-chain hydrogen bonding between carbonyl oxygens and amide hydrogens. Thus, the correct answer (and false statement) is that each is stabilized by interchain hydrogen bonds.
Alpha-helices and beta-sheets are secondary structure motifs that occur when sequences of amino acids are linked by hydrogen bonds. These secondary structures are an integral part of globular proteins, such as hemoglobin. Alpha-helices resemble a coiled spring, with hydrogen bonding occurring in an intra-chain arrangement between carbonyl oxygens and amide hydrogens that is parallel to the central axis. Beta sheets, on the other hand, may have either inter- or intra-chain hydrogen bonding between carbonyl oxygens and amide hydrogens. Thus, the correct answer (and false statement) is that each is stabilized by interchain hydrogen bonds.
Compare your answer with the correct one above
Which of the following describes the folding of soluble globular proteins?
Which of the following describes the folding of soluble globular proteins?
Globular proteins are representative of the quaternary structure of a class of proteins, an example of which is hemoglobin. In a soluble molecule the surface of the molecule must interact with water. Any hydrophobic portions of the molecule must remain internal and away from water, while hydrophilic portions will remain on the exterior portion interacting with water molecules. A soluble globular protein is folded so as to minimize the energy of the system. Thus, the correct answer is that most hydrophobic amino acids are internal, away from solvent water.
Globular proteins are representative of the quaternary structure of a class of proteins, an example of which is hemoglobin. In a soluble molecule the surface of the molecule must interact with water. Any hydrophobic portions of the molecule must remain internal and away from water, while hydrophilic portions will remain on the exterior portion interacting with water molecules. A soluble globular protein is folded so as to minimize the energy of the system. Thus, the correct answer is that most hydrophobic amino acids are internal, away from solvent water.
Compare your answer with the correct one above
The term "denaturation," when used in conjunction with proteins or nucleic acids, refers to a change in structural characteristics primarily due to __________.
The term "denaturation," when used in conjunction with proteins or nucleic acids, refers to a change in structural characteristics primarily due to __________.
The denaturation of proteins and nucleic acids occurs due to the disruption of non-covalent bonds, especially hydrogen bonds. In the case of nucleic acids, covalent bonds can be disrupted by specific enzymes, but this is not a form of denaturation. Changes in the primary structure of nucleic acids and proteins simply result in the net production of different proteins due to sequential changes of amino acids and nucleotides, not denaturation. Toxic compounds can interfere with nucleic acid and protein formation, but they do so by interrupting the non-covalent forces of each, such as hydrophobic forces and hydrogen bonding. Thus, the correct answer is the disruption of non-covalent bonds.
The denaturation of proteins and nucleic acids occurs due to the disruption of non-covalent bonds, especially hydrogen bonds. In the case of nucleic acids, covalent bonds can be disrupted by specific enzymes, but this is not a form of denaturation. Changes in the primary structure of nucleic acids and proteins simply result in the net production of different proteins due to sequential changes of amino acids and nucleotides, not denaturation. Toxic compounds can interfere with nucleic acid and protein formation, but they do so by interrupting the non-covalent forces of each, such as hydrophobic forces and hydrogen bonding. Thus, the correct answer is the disruption of non-covalent bonds.
Compare your answer with the correct one above
You are given the amino acid sequence Ala-Gly-His-Tyr. This is an example of which level of protein structure?
You are given the amino acid sequence Ala-Gly-His-Tyr. This is an example of which level of protein structure?
Primary protein structure refers to the linear sequence of amino acids, which is the information given in this question. Secondary structure includes interactions between nearby parts of the linear chain, and includes the common examples of alpha-helices and beta-pleated sheets. Tertiary structure is the protein's three-dimensional shape, and quaternary structure refers to interactions between tertiary subunits.
Primary protein structure refers to the linear sequence of amino acids, which is the information given in this question. Secondary structure includes interactions between nearby parts of the linear chain, and includes the common examples of alpha-helices and beta-pleated sheets. Tertiary structure is the protein's three-dimensional shape, and quaternary structure refers to interactions between tertiary subunits.
Compare your answer with the correct one above
Hemoglobin is the principal oxygen-carrying protein in humans. It exists within erythrocytes, and binds up to four diatomic oxygen molecules simultaneously. Hemoglobin functions to maximize oxygen delivery to tissues, while simultaneously maximizing oxygen absorption in the lungs. Hemoglobin thus has a fundamentally contradictory set of goals. It must at once be opitimized to absorb oxygen, and to offload oxygen. Natural selection has overcome this apparent contradiction by making hemoglobin exquisitely sensitive to conditions in its microenvironment.
One way in which hemoglobin accomplishes its goals is through the phenomenon of cooperativity. Cooperativity refers to the ability of hemoglobin to change its oxygen binding behavior as a function of how many other oxygen atoms are bound to the molecule.
Fetal hemoglobin shows a similar pattern of cooperativity, but has unique binding characteristics relative to adult hemoglobin. Fetal hemoglobin reaches higher saturation at lower oxygen partial pressure.
Because of cooperativity, adult and fetal oxygen-hemoglobin dissociation curves appear as follows.

Beyond its ability to carry oxygen, hemoglobin is also effective as a blood buffer. The general reaction for the blood buffer system of hemoglobin is given below.
H+ + HbO2 ←→ H+Hb + O2
What portions of the amino acids in hemoglobin likely mediate the differences in the binding and non-binding regions of hemoglobin?
Hemoglobin is the principal oxygen-carrying protein in humans. It exists within erythrocytes, and binds up to four diatomic oxygen molecules simultaneously. Hemoglobin functions to maximize oxygen delivery to tissues, while simultaneously maximizing oxygen absorption in the lungs. Hemoglobin thus has a fundamentally contradictory set of goals. It must at once be opitimized to absorb oxygen, and to offload oxygen. Natural selection has overcome this apparent contradiction by making hemoglobin exquisitely sensitive to conditions in its microenvironment.
One way in which hemoglobin accomplishes its goals is through the phenomenon of cooperativity. Cooperativity refers to the ability of hemoglobin to change its oxygen binding behavior as a function of how many other oxygen atoms are bound to the molecule.
Fetal hemoglobin shows a similar pattern of cooperativity, but has unique binding characteristics relative to adult hemoglobin. Fetal hemoglobin reaches higher saturation at lower oxygen partial pressure.
Because of cooperativity, adult and fetal oxygen-hemoglobin dissociation curves appear as follows.

Beyond its ability to carry oxygen, hemoglobin is also effective as a blood buffer. The general reaction for the blood buffer system of hemoglobin is given below.
H+ + HbO2 ←→ H+Hb + O2
What portions of the amino acids in hemoglobin likely mediate the differences in the binding and non-binding regions of hemoglobin?
Side chains are the principal point of variety in amino acids. When amino acids contribute to differential functions of proteins, it is typically the side chains that mediate how the amino acids behave in specific environments. All other answers are static components of amino acids and proteins, and would not change regardless of position in the protein. As such, they would not be capable of explaining regional differences.
Side chains are the principal point of variety in amino acids. When amino acids contribute to differential functions of proteins, it is typically the side chains that mediate how the amino acids behave in specific environments. All other answers are static components of amino acids and proteins, and would not change regardless of position in the protein. As such, they would not be capable of explaining regional differences.
Compare your answer with the correct one above
Hemoglobin is the principal oxygen-carrying protein in humans. It exists within erythrocytes, and binds up to four diatomic oxygen molecules simultaneously. Hemoglobin functions to maximize oxygen delivery to tissues, while simultaneously maximizing oxygen absorption in the lungs. Hemoglobin thus has a fundamentally contradictory set of goals. It must at once be opitimized to absorb oxygen, and to offload oxygen. Natural selection has overcome this apparent contradiction by making hemoglobin exquisitely sensitive to conditions in its microenvironment.
One way in which hemoglobin accomplishes its goals is through the phenomenon of cooperativity. Cooperativity refers to the ability of hemoglobin to change its oxygen binding behavior as a function of how many other oxygen atoms are bound to the molecule.
Fetal hemoglobin shows a similar pattern of cooperativity, but has unique binding characteristics relative to adult hemoglobin. Fetal hemoglobin reaches higher saturation at lower oxygen partial pressure.
Because of cooperativity, adult and fetal oxygen-hemoglobin dissociation curves appear as follows.

Beyond its ability to carry oxygen, hemoglobin is also effective as a blood buffer. The general reaction for the blood buffer system of hemoglobin is given below.
H+ + HbO2 ←→ H+Hb + O2
Because hemoglobin can act as a buffer in blood, it helps keep the pH constant. Which of the following portions of an amino acid can change with pH change?
Hemoglobin is the principal oxygen-carrying protein in humans. It exists within erythrocytes, and binds up to four diatomic oxygen molecules simultaneously. Hemoglobin functions to maximize oxygen delivery to tissues, while simultaneously maximizing oxygen absorption in the lungs. Hemoglobin thus has a fundamentally contradictory set of goals. It must at once be opitimized to absorb oxygen, and to offload oxygen. Natural selection has overcome this apparent contradiction by making hemoglobin exquisitely sensitive to conditions in its microenvironment.
One way in which hemoglobin accomplishes its goals is through the phenomenon of cooperativity. Cooperativity refers to the ability of hemoglobin to change its oxygen binding behavior as a function of how many other oxygen atoms are bound to the molecule.
Fetal hemoglobin shows a similar pattern of cooperativity, but has unique binding characteristics relative to adult hemoglobin. Fetal hemoglobin reaches higher saturation at lower oxygen partial pressure.
Because of cooperativity, adult and fetal oxygen-hemoglobin dissociation curves appear as follows.

Beyond its ability to carry oxygen, hemoglobin is also effective as a blood buffer. The general reaction for the blood buffer system of hemoglobin is given below.
H+ + HbO2 ←→ H+Hb + O2
Because hemoglobin can act as a buffer in blood, it helps keep the pH constant. Which of the following portions of an amino acid can change with pH change?
All three portions can change with pH. The amino end can take on an extra proton to become positively charged, the carboxy end can lose a proton and take on a negative charge, and the side chain can do either depending on its structure. An amino acid with both a positively charged amino end and a negatively charged carboxy end is called a zwitterion.
All three portions can change with pH. The amino end can take on an extra proton to become positively charged, the carboxy end can lose a proton and take on a negative charge, and the side chain can do either depending on its structure. An amino acid with both a positively charged amino end and a negatively charged carboxy end is called a zwitterion.
Compare your answer with the correct one above
Cryptosporidium is a genus of gastrointestinal parasite that infects the intestinal epithelium of mammals. Cryptosporidium is water-borne, and is an apicomplexan parasite. This phylum also includes Plasmodium, Babesia, and Toxoplasma.
Apicomplexans are unique due to their apicoplast, an apical organelle that helps penetrate mammalian epithelium. In the case of cryptosporidium, there is an interaction between the surface proteins of mammalian epithelial tissue and those of the apical portion of the cryptosporidium infective stage, or oocyst. A scientist is conducting an experiment to test the hypothesis that the oocyst secretes a peptide compound that neutralizes intestinal defense cells. These defense cells are resident in the intestinal epithelium, and defend the tissue by phagocytizing the oocysts.
She sets up the following experiment:
As the neutralizing compound was believed to be secreted by the oocyst, the scientist collected oocysts onto growth media. The oocysts were grown among intestinal epithelial cells, and then the media was collected. The media was then added to another plate where Toxoplasma gondii was growing with intestinal epithelial cells. A second plate of Toxoplasma gondii was grown with the same type of intestinal epithelium, but no oocyst-sourced media was added.
After conducting the experiment described in the passage, the scientist attempts to determine the overall three dimensional shape of the protein toxin secreted by the cryptosporidium oocysts. What is the scientist investigating?
Cryptosporidium is a genus of gastrointestinal parasite that infects the intestinal epithelium of mammals. Cryptosporidium is water-borne, and is an apicomplexan parasite. This phylum also includes Plasmodium, Babesia, and Toxoplasma.
Apicomplexans are unique due to their apicoplast, an apical organelle that helps penetrate mammalian epithelium. In the case of cryptosporidium, there is an interaction between the surface proteins of mammalian epithelial tissue and those of the apical portion of the cryptosporidium infective stage, or oocyst. A scientist is conducting an experiment to test the hypothesis that the oocyst secretes a peptide compound that neutralizes intestinal defense cells. These defense cells are resident in the intestinal epithelium, and defend the tissue by phagocytizing the oocysts.
She sets up the following experiment:
As the neutralizing compound was believed to be secreted by the oocyst, the scientist collected oocysts onto growth media. The oocysts were grown among intestinal epithelial cells, and then the media was collected. The media was then added to another plate where Toxoplasma gondii was growing with intestinal epithelial cells. A second plate of Toxoplasma gondii was grown with the same type of intestinal epithelium, but no oocyst-sourced media was added.
After conducting the experiment described in the passage, the scientist attempts to determine the overall three dimensional shape of the protein toxin secreted by the cryptosporidium oocysts. What is the scientist investigating?
The tertiary structure of a polypeptide chain is defined as the overall shape. It is determined by the primary structure, or sequence of amino acids, and the secondary structures in the polypeptide, which are usually composed of beta-sheet or alpha-helix conformations.
The tertiary structure of a polypeptide chain is defined as the overall shape. It is determined by the primary structure, or sequence of amino acids, and the secondary structures in the polypeptide, which are usually composed of beta-sheet or alpha-helix conformations.
Compare your answer with the correct one above
Which level of protein structure is stabilized primarily by hydrogen bonding?
Which level of protein structure is stabilized primarily by hydrogen bonding?
Secondary structure is observed when the primary sequence of amino acids conforms into either alpha-helices and/or beta-pleated sheets. These conformations of the polypeptide chain are stabilized by hydrogen bonding alone.
Primary structure is determined by peptide bonds. Tertiary structure is determined by disulfide bonds and hydrophobic interactions. Quaternary structure is determined by interactions between multiple subunits.
Secondary structure is observed when the primary sequence of amino acids conforms into either alpha-helices and/or beta-pleated sheets. These conformations of the polypeptide chain are stabilized by hydrogen bonding alone.
Primary structure is determined by peptide bonds. Tertiary structure is determined by disulfide bonds and hydrophobic interactions. Quaternary structure is determined by interactions between multiple subunits.
Compare your answer with the correct one above
Collagen, the most abundant protein in the body, is an example of what type of protein?
Collagen, the most abundant protein in the body, is an example of what type of protein?
Collagen is a structural protein that adds significant strength and resilience to the skin, tendons, and ligaments. Structural proteins, including collagen, also fall under the category of fibrous proteins. Globular proteins, in contrast, usually act as enzymes in the body or transport channels in the membrane.
Peripheral proteins are a type of globular protein found adjacent to the membrane, while integral proteins are transmembrane globular proteins.
Collagen is a structural protein that adds significant strength and resilience to the skin, tendons, and ligaments. Structural proteins, including collagen, also fall under the category of fibrous proteins. Globular proteins, in contrast, usually act as enzymes in the body or transport channels in the membrane.
Peripheral proteins are a type of globular protein found adjacent to the membrane, while integral proteins are transmembrane globular proteins.
Compare your answer with the correct one above
Amino acids are joined together to form polypeptides. Each amino acid is attached to another by a peptide bond.
What functional group is created when amino acids are joined together?
Amino acids are joined together to form polypeptides. Each amino acid is attached to another by a peptide bond.
What functional group is created when amino acids are joined together?
Polypeptide formation involves the C-terminus of one amino acid attaching to the N-terminus of another. This polymerization results in a dipeptide with the byproduct of one water molecule. The newfound combination results in a carbonyl being attached to a nitrogen. This functional group is called an amide.
Polypeptide formation involves the C-terminus of one amino acid attaching to the N-terminus of another. This polymerization results in a dipeptide with the byproduct of one water molecule. The newfound combination results in a carbonyl being attached to a nitrogen. This functional group is called an amide.
Compare your answer with the correct one above
In 2013, scientists linked a cellular response called the unfolded protein response (UPR) to a series of neurodegenerative diseases, including such major health issues as Parkinson’s and Alzheimer’s Disease. According to their work, the unfolded protein response is a reduction in translation as a result of a series of enzymes that modify a translation initiation factor, eIF2, as below:

In the above sequence, the unfolded protein sensor binds to unfolded protein, such as the pathogenic amyloid-beta found in the brains of Alzheimer’s Disease patients. This sensor then phosphorylates PERK, or protein kinase RNA-like endoplasmic reticulum kinase. This leads to downstream effects on eIF2, inhibition of which represses translation. It is thought that symptoms of neurodegenerative disease may be a result of this reduced translation.
Which of the following is the LEAST important force that promotes protein folding?
In 2013, scientists linked a cellular response called the unfolded protein response (UPR) to a series of neurodegenerative diseases, including such major health issues as Parkinson’s and Alzheimer’s Disease. According to their work, the unfolded protein response is a reduction in translation as a result of a series of enzymes that modify a translation initiation factor, eIF2, as below:

In the above sequence, the unfolded protein sensor binds to unfolded protein, such as the pathogenic amyloid-beta found in the brains of Alzheimer’s Disease patients. This sensor then phosphorylates PERK, or protein kinase RNA-like endoplasmic reticulum kinase. This leads to downstream effects on eIF2, inhibition of which represses translation. It is thought that symptoms of neurodegenerative disease may be a result of this reduced translation.
Which of the following is the LEAST important force that promotes protein folding?
Metallic bonding adheres to the "nuclei in a sea of electrons" model that explains the malleability, conductivity, and ductility of metals. Though some proteins (like hemoglobin) rely on a metallic compound, metallic interactions do not dictate the majority of protein folding interactions.
Proteins have a non-metal backbone, and are more dependent on dipole, hydrogen, covalent, and van der Waals forces to dictate their conformation.
Metallic bonding adheres to the "nuclei in a sea of electrons" model that explains the malleability, conductivity, and ductility of metals. Though some proteins (like hemoglobin) rely on a metallic compound, metallic interactions do not dictate the majority of protein folding interactions.
Proteins have a non-metal backbone, and are more dependent on dipole, hydrogen, covalent, and van der Waals forces to dictate their conformation.
Compare your answer with the correct one above
In the crusade to create a vaccine for Poliomyelitis, Jonas Salk and Albert Sabin created two separate vaccines that proved to be successful in preventing Polio onset.
The Salk vaccine, which is given by standard injection, contained virus particles inactivated by an organic solvent. This method has the advantage of inactivating each of the three Polio strains with no bias.
Albert Sabin's vaccine, given by oral inoculation via sugar water, contained live virus particles that had been genetically attenuated. With this method, each of the three Polio strains acquired separate mutations that made them unable to infect the human host cells. Strain 2 in particular contained one single nucleotide polymorphism in the internal ribosomal entry site (IRES) that prevented successful viral replication.
The organic solvent used to inactivate the Poliovirus in the Salk vaccine significantly alters the viral capsid. For the purposes of this question, let us assume that the capsid proteins are bound together by multiple di-sulfide bonds. Given this information, which of the solvents listed below would be most effective in disrupting the Poliovirus capsid?
In the crusade to create a vaccine for Poliomyelitis, Jonas Salk and Albert Sabin created two separate vaccines that proved to be successful in preventing Polio onset.
The Salk vaccine, which is given by standard injection, contained virus particles inactivated by an organic solvent. This method has the advantage of inactivating each of the three Polio strains with no bias.
Albert Sabin's vaccine, given by oral inoculation via sugar water, contained live virus particles that had been genetically attenuated. With this method, each of the three Polio strains acquired separate mutations that made them unable to infect the human host cells. Strain 2 in particular contained one single nucleotide polymorphism in the internal ribosomal entry site (IRES) that prevented successful viral replication.
The organic solvent used to inactivate the Poliovirus in the Salk vaccine significantly alters the viral capsid. For the purposes of this question, let us assume that the capsid proteins are bound together by multiple di-sulfide bonds. Given this information, which of the solvents listed below would be most effective in disrupting the Poliovirus capsid?
The answer is 2-mercaptoethanol because it contains strong reducing groups that are capable of reducing the di-sulfide bonds.
Dimethyl sulfoxide (DMSO), methanol, and ethanol do not contain reducing groups capable of breaking di-sulfide bonds, if at all.
The answer is 2-mercaptoethanol because it contains strong reducing groups that are capable of reducing the di-sulfide bonds.
Dimethyl sulfoxide (DMSO), methanol, and ethanol do not contain reducing groups capable of breaking di-sulfide bonds, if at all.
Compare your answer with the correct one above
An enzyme that cleaves disulfide bridges would most disrupt a protein containing which amino acid sequence?
An enzyme that cleaves disulfide bridges would most disrupt a protein containing which amino acid sequence?
Disulfide bridges are made between two cysteine amino acids. An enzyme that cleaves disulfide bonds would disrupt a protein containing the most cysteine residues; therefore, Tyr–Cys–Cys–Thr–Val–Leu is the correct answer.
Disulfide bridges are made between two cysteine amino acids. An enzyme that cleaves disulfide bonds would disrupt a protein containing the most cysteine residues; therefore, Tyr–Cys–Cys–Thr–Val–Leu is the correct answer.
Compare your answer with the correct one above
Proteins can have a maximum of four levels of structure: primary, secondary, tertiary, and quaternary. Although the proteins can spontaneously fold to a functional conformation, there are a variety of denaturing agents that can be used to disrupt the folding strategies of proteins. Mercaptoethanol is an example of a protein denaturing agent; its mechanism for dismantling proteins is to disrupt the disulfide bonds found in the protein. When urea is introduced to a protein, the hydrogen bonds holding the protein together are disrupted. Heat can also be considered a denaturing agent, which has the potential to disrupt all intermolecular interactions in a protein.
Which of the following levels of structure in a protein would not be disrupted by the introduction of mercaptoethanol?
Proteins can have a maximum of four levels of structure: primary, secondary, tertiary, and quaternary. Although the proteins can spontaneously fold to a functional conformation, there are a variety of denaturing agents that can be used to disrupt the folding strategies of proteins. Mercaptoethanol is an example of a protein denaturing agent; its mechanism for dismantling proteins is to disrupt the disulfide bonds found in the protein. When urea is introduced to a protein, the hydrogen bonds holding the protein together are disrupted. Heat can also be considered a denaturing agent, which has the potential to disrupt all intermolecular interactions in a protein.
Which of the following levels of structure in a protein would not be disrupted by the introduction of mercaptoethanol?
When discussing the secondary structure of a protein, you can assume that the only forces that are relevant are the hydrogen bonds between the carbonyl oxygen of one amino acid, and the hydrogen on the amino group of another. Because hydrogen bonds are the only intermolecular interaction involved in secondary structure, mercaptoethanol would not affect the secondary structure.
Disulfide bonds are generally integral to defining the tertiary structure of a protein; thus, mercaptoethanol would affect the tertiary structure (and subsequent quaternary structure) of a protein.
When discussing the secondary structure of a protein, you can assume that the only forces that are relevant are the hydrogen bonds between the carbonyl oxygen of one amino acid, and the hydrogen on the amino group of another. Because hydrogen bonds are the only intermolecular interaction involved in secondary structure, mercaptoethanol would not affect the secondary structure.
Disulfide bonds are generally integral to defining the tertiary structure of a protein; thus, mercaptoethanol would affect the tertiary structure (and subsequent quaternary structure) of a protein.
Compare your answer with the correct one above
Proteins can have a maximum of four levels of structure: primary, secondary, tertiary, and quaternary. Although the proteins can spontaneously fold to a functional conformation, there are a variety of denaturing agents that can be used to disrupt the folding strategies of proteins. Mercaptoethanol is an example of a protein denaturing agent; its mechanism for dismantling proteins is to disrupt the disulfide bonds found in the protein. When urea is introduced to a protein, the hydrogen bonds holding the protein together are disrupted. Heat can also be considered a denaturing agent, which has the potential to disrupt all intermolecular interactions in a protein.
Which of the following levels of structure would not be affected by urea?
Proteins can have a maximum of four levels of structure: primary, secondary, tertiary, and quaternary. Although the proteins can spontaneously fold to a functional conformation, there are a variety of denaturing agents that can be used to disrupt the folding strategies of proteins. Mercaptoethanol is an example of a protein denaturing agent; its mechanism for dismantling proteins is to disrupt the disulfide bonds found in the protein. When urea is introduced to a protein, the hydrogen bonds holding the protein together are disrupted. Heat can also be considered a denaturing agent, which has the potential to disrupt all intermolecular interactions in a protein.
Which of the following levels of structure would not be affected by urea?
Urea is used to denature proteins by interrupting hydrogen bonds. Hydrogen bonds are found in all levels beyond the primary structure, so all of the above levels will be affected by an introduction of urea.
Hydrogen bonds are particularly important to defining secondary structure, as it is these forces that create alpha-helices and beta-pleated sheets. Without proper secondary structure, tertiary and quaternary development will also be disrupted.
Urea is used to denature proteins by interrupting hydrogen bonds. Hydrogen bonds are found in all levels beyond the primary structure, so all of the above levels will be affected by an introduction of urea.
Hydrogen bonds are particularly important to defining secondary structure, as it is these forces that create alpha-helices and beta-pleated sheets. Without proper secondary structure, tertiary and quaternary development will also be disrupted.
Compare your answer with the correct one above
Which of these choices correctly pairs the level of protein structure with an example of that level of structure?
Which of these choices correctly pairs the level of protein structure with an example of that level of structure?
There are four distinct levels of protein structure: primary, secondary, tertiary, and quaternary. Primary structure refers to the actual sequence of amino acids, like Ala-Met-Gly-Trp, which are held together by peptide bonds. Secondary structure, which includes alpha-helices and beta-pleated sheets, is the local three-dimensional shape created by hydrogen bonding. Tertiary structure is the overall shape of the protein subunit, caused by more distant interactions. Disulfide bonds (bonds between the sulfur atoms of two cysteine amino acids) are an example of tertiary structure. Finally, quaternary structure involves interactions between the peptide subunits of a larger protein complex.
There are four distinct levels of protein structure: primary, secondary, tertiary, and quaternary. Primary structure refers to the actual sequence of amino acids, like Ala-Met-Gly-Trp, which are held together by peptide bonds. Secondary structure, which includes alpha-helices and beta-pleated sheets, is the local three-dimensional shape created by hydrogen bonding. Tertiary structure is the overall shape of the protein subunit, caused by more distant interactions. Disulfide bonds (bonds between the sulfur atoms of two cysteine amino acids) are an example of tertiary structure. Finally, quaternary structure involves interactions between the peptide subunits of a larger protein complex.
Compare your answer with the correct one above
Hypersensitivity reactions occur when body tissues are affected by an abnormal immune reaction. The result is damage to normal tissues and clinical illness. A peanut allergy is an example of a hypersensitivity reaction, but there are three additional broad classes.
One class involves the abnormal production or deposition of antibodies. Antibodies are B-cell derived molecules that normally adhere to pathogens, rendering them unable to continue an infection. When antibodies are produced against normal tissues, however, disease can result. Figure 1 depicts a schematic structure of an antibody.
Antibodies can be divided into two peptide chains: heavy and light. Heavy chains form the backbone of the antibody, and are attached to light chains via covalent bonding. Each heavy and light chain is then further divided into constant and variable regions. Variable regions exhibit molecular variety, generating a unique chemical identity for each antibody. These unique patterns help guarantee that the body can produce antibodies to recognize many possible molecular patterns on invading pathogens.
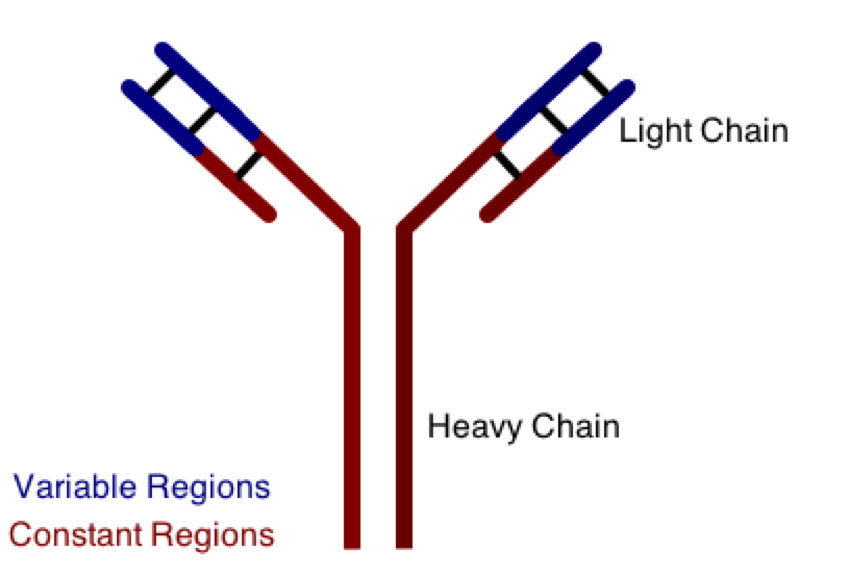
The polypeptides that make up the heavy and light chains of antibodies are most likely connected by covalent bridges involving atoms of which element?
Hypersensitivity reactions occur when body tissues are affected by an abnormal immune reaction. The result is damage to normal tissues and clinical illness. A peanut allergy is an example of a hypersensitivity reaction, but there are three additional broad classes.
One class involves the abnormal production or deposition of antibodies. Antibodies are B-cell derived molecules that normally adhere to pathogens, rendering them unable to continue an infection. When antibodies are produced against normal tissues, however, disease can result. Figure 1 depicts a schematic structure of an antibody.
Antibodies can be divided into two peptide chains: heavy and light. Heavy chains form the backbone of the antibody, and are attached to light chains via covalent bonding. Each heavy and light chain is then further divided into constant and variable regions. Variable regions exhibit molecular variety, generating a unique chemical identity for each antibody. These unique patterns help guarantee that the body can produce antibodies to recognize many possible molecular patterns on invading pathogens.

The polypeptides that make up the heavy and light chains of antibodies are most likely connected by covalent bridges involving atoms of which element?
Covalent bridges can be found in organic molecules, linking one region of the molecule to another. These bridges are almost invariably disulfide linkages, in which two sulfur atoms form a covalent linkage that provides a great deal of stability between peptide chains. Disulfide bridges are commonly involved in protein tertiary structure and other organic structural linkages, such as the joining of the heavy and light chains in antibodies.
Covalent bridges can be found in organic molecules, linking one region of the molecule to another. These bridges are almost invariably disulfide linkages, in which two sulfur atoms form a covalent linkage that provides a great deal of stability between peptide chains. Disulfide bridges are commonly involved in protein tertiary structure and other organic structural linkages, such as the joining of the heavy and light chains in antibodies.
Compare your answer with the correct one above
Which of the following is an example of the secondary structure of a protein?
Which of the following is an example of the secondary structure of a protein?
By definition, the secondary structure of a protein is the hydrogen bonding between the amine and carbonyl groups in the amino acid chain. This usually occurs in the form of alpha-helices or beta-pleated sheets.
The linear sequence of the amino acids formed by peptide bonds is the primary protein structure. Interactions of R groups determines the tertiary structure. These interactions can be in the form of disulfide bonds, hydrogen bonding, or hydrophobic interactions.
By definition, the secondary structure of a protein is the hydrogen bonding between the amine and carbonyl groups in the amino acid chain. This usually occurs in the form of alpha-helices or beta-pleated sheets.
The linear sequence of the amino acids formed by peptide bonds is the primary protein structure. Interactions of R groups determines the tertiary structure. These interactions can be in the form of disulfide bonds, hydrogen bonding, or hydrophobic interactions.
Compare your answer with the correct one above
Hypersensitivity reactions occur when body tissues are affected by an abnormal immune reaction. The result is damage to normal tissues and clinical illness. A peanut allergy is an example of a hypersensitivity reaction, but there are three additional broad classes.
One class involves the abnormal production or deposition of antibodies. Antibodies are B-cell derived molecules that normally adhere to pathogens, rendering them unable to continue an infection. When antibodies are produced against normal tissues, however, disease can result. Figure 1 depicts a schematic structure of an antibody.
Antibodies can be divided into two peptide chains: heavy and light. Heavy chains form the backbone of the antibody, and are attached to light chains via covalent bonding. Each heavy and light chain is then further divided into constant and variable regions. Variable regions exhibit molecular variety, generating a unique chemical identity for each antibody. These unique patterns help guarantee that the body can produce antibodies to recognize many possible molecular patterns on invading pathogens.

Antibodies are made of proteins, which form one of the broad classes of biological macromolecules. A glycoprotein is different from other kinds of proteins principally because __________.
Hypersensitivity reactions occur when body tissues are affected by an abnormal immune reaction. The result is damage to normal tissues and clinical illness. A peanut allergy is an example of a hypersensitivity reaction, but there are three additional broad classes.
One class involves the abnormal production or deposition of antibodies. Antibodies are B-cell derived molecules that normally adhere to pathogens, rendering them unable to continue an infection. When antibodies are produced against normal tissues, however, disease can result. Figure 1 depicts a schematic structure of an antibody.
Antibodies can be divided into two peptide chains: heavy and light. Heavy chains form the backbone of the antibody, and are attached to light chains via covalent bonding. Each heavy and light chain is then further divided into constant and variable regions. Variable regions exhibit molecular variety, generating a unique chemical identity for each antibody. These unique patterns help guarantee that the body can produce antibodies to recognize many possible molecular patterns on invading pathogens.

Antibodies are made of proteins, which form one of the broad classes of biological macromolecules. A glycoprotein is different from other kinds of proteins principally because __________.
The prefix "glyco-" indicates that some substrate has had a carbohydrate moiety added to its structure. Glycolipids are thus lipids bound to saccharide units, and glycoproteins are proteins bound to saccharide units.
The prefix "glyco-" indicates that some substrate has had a carbohydrate moiety added to its structure. Glycolipids are thus lipids bound to saccharide units, and glycoproteins are proteins bound to saccharide units.
Compare your answer with the correct one above