Other Reactions
Practice Questions
MCAT Biology › Other Reactions
Carbonic anhydrase is a very important enzyme that is utilized by the body. The enzyme catalyzes the following reaction:
A class of drugs that inhibits this enzyme is carbonic anhydrase inhibitors (eg. acetazolamide, brinzolamide, dorzolamide). These drugs are commonly prescribed in patients with glaucoma, hypertension, heart failure, high altitude sickness and for the treatment of basic drugs overdose.
In patients with hypertension, carbonic anhydrase inhibitors will prevent the reabsorption of sodium chloride 
When mountain climbing, the atmospheric pressure is lowered as the altitude increases. As a result of less oxygen into the lungs, ventilation increases. From the equation above, hyperventilation will result in more 
Carbonic anhydrase inhibitors are useful in patients with a drug overdose that is acidic. The lumen of the collecting tubule is nonpolar. Due to the lumen's characteristic, molecules that are also nonpolar and uncharged are able to cross the membrane and re-enter the circulatory system. Since carbonic anhydrase inhibitors alkalize the urine, acidic molecules stay in a charged state.
When a patient is not ventilating efficiently, the kidneys try to compensate by producing more bicarbonate to buffer the decreasing pH of the blood due to the buildup of 

I. Increase 
II. Increase 
III. Increase metabolism
Carbonic anhydrase is a very important enzyme that is utilized by the body. The enzyme catalyzes the following reaction:
A class of drugs that inhibits this enzyme is carbonic anhydrase inhibitors (eg. acetazolamide, brinzolamide, dorzolamide). These drugs are commonly prescribed in patients with glaucoma, hypertension, heart failure, high altitude sickness and for the treatment of basic drugs overdose.
In patients with hypertension, carbonic anhydrase inhibitors will prevent the reabsorption of sodium chloride 
When mountain climbing, the atmospheric pressure is lowered as the altitude increases. As a result of less oxygen into the lungs, ventilation increases. From the equation above, hyperventilation will result in more 
Carbonic anhydrase inhibitors are useful in patients with a drug overdose that is acidic. The lumen of the collecting tubule is nonpolar. Due to the lumen's characteristic, molecules that are also nonpolar and uncharged are able to cross the membrane and re-enter the circulatory system. Since carbonic anhydrase inhibitors alkalize the urine, acidic molecules stay in a charged state.
Based on the passage, which of the following statements, if true, will contradict the effectiveness of carbonic anhydrase inhibitors as a treatment?
Which of the following is not capable of oxidizing a secondary alcohol to a ketone?
Carbonic anhydrase is a very important enzyme that is utilized by the body. The enzyme catalyzes the following reaction:
A class of drugs that inhibits this enzyme is carbonic anhydrase inhibitors (eg. acetazolamide, brinzolamide, dorzolamide). These drugs are commonly prescribed in patients with glaucoma, hypertension, heart failure, high altitude sickness and for the treatment of basic drugs overdose.
In patients with hypertension, carbonic anhydrase inhibitors will prevent the reabsorption of sodium chloride 
When mountain climbing, the atmospheric pressure is lowered as the altitude increases. As a result of less oxygen into the lungs, ventilation increases. From the equation above, hyperventilation will result in more 
Carbonic anhydrase inhibitors are useful in patients with a drug overdose that is acidic. The lumen of the collecting tubule is nonpolar. Due to the lumen's characteristic, molecules that are also nonpolar and uncharged are able to cross the membrane and re-enter the circulatory system. Since carbonic anhydrase inhibitors alkalize the urine, acidic molecules stay in a charged state.
A patient overdosed on an unknown drug. Two hours after being admitted into the hospital, acetazolamide was administered. A realtime measurement of the drug's concentration in the patient's blood was taken the moment the patient was admitted every 30 minutes. Which of the following best explains the lab results shown?
Carbonic anhydrase is a very important enzyme that is utilized by the body. The enzyme catalyzes the following reaction:
A class of drugs that inhibits this enzyme is carbonic anhydrase inhibitors (eg. acetazolamide, brinzolamide, dorzolamide). These drugs are commonly prescribed in patients with glaucoma, hypertension, heart failure, high altitude sickness and for the treatment of basic drugs overdose.
In patients with hypertension, carbonic anhydrase inhibitors will prevent the reabsorption of sodium chloride 
When mountain climbing, the atmospheric pressure is lowered as the altitude increases. As a result of less oxygen into the lungs, ventilation increases. From the equation above, hyperventilation will result in more 
Carbonic anhydrase inhibitors are useful in patients with a drug overdose that is acidic. The lumen of the collecting tubule is nonpolar. Due to the lumen's characteristic, molecules that are also nonpolar and uncharged are able to cross the membrane and re-enter the circulatory system. Since carbonic anhydrase inhibitors alkalize the urine, acidic molecules stay in a charged state.
Glaucoma involves the pressure within the eye increasing to a dangerously high level due to improper draining of the vitreous humor. Blindness usually follows due to damage to the nerves. How might administering a carbonic anhydrase inhibitor help in patients with glaucoma?
Which of the following is an example of an anabolic reaction in the body?
In the human body, glycerol and three fatty acids can act together to form triacyl glycerol in a process known as esterification. The reverse of this process is known as __________.
Triacylglycerols are broken down in the body by lipases. In the presence of water, lipase is used to break down the triacylglycerol into a glycerol molecule and three fatty acids. Based on this information, the breakdown of a triacylglycerol is the opposite of which reaction?
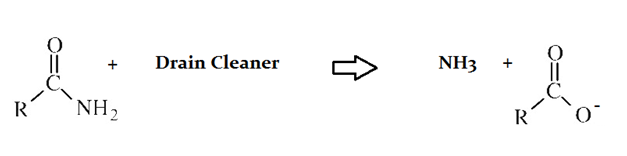
Drain cleaners a common household staple, used to open clogged drains in bathtubs and sinks. Human hair is a common culprit that clogs pipes, and hair is made predominately of protein. Drain cleaners are effective at breaking down proteins that have accumulated in plumbing. Drain cleaners can be either acidic or basic, and are also effective at breaking down fats that have accumulated with proteins.
A typical reaction—reaction 1—which would be expected for a drain cleaner on contact with human hair, would be as follows in an aqueous solution:
Another reaction that may occur, reaction 2, would take place as follows in an aqueous solution:
An alcohol reacts with the protein reactant in Reaction 2. Which atom in the protein reactant is likely to be the site of a nucleophilic attack?
Which of these is a dehydration synthesis reaction?
I. Formation of a disulfide bond between two cysteine residues
II. Carboxylation of a carbohydrate
III. Formation of an amide bond
IV. Splitting of dipeptides into amino acids
V. Addition of hydrogen to an unsaturated fatty acid