Functional Groups and Properties
Practice Questions
MCAT Biology › Functional Groups and Properties
Ephedrine (shown below) contains what type of amine?

Carboxylic acids typically have higher boiling points than aldehydes and ketones. This is because carboxylic acids have which of the following properties?
Which of the following compounds will be the most reactive with an alcohol to form an ester product?
In the reaction scheme below, compound A is a(n) __________ and compound B is a(n) __________.

Compound A, shown below, contains an example of what type of functional group?

Drain cleaners a common household staple, used to open clogged drains in bathtubs and sinks. Human hair is a common culprit that clogs pipes, and hair is made predominately of protein. Drain cleaners are effective at breaking down proteins that have accumulated in plumbing. Drain cleaners can be either acidic or basic, and are also effective at breaking down fats that have accumulated with proteins.
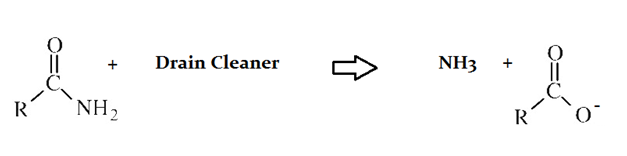
A typical reaction—reaction 1—which would be expected for a drain cleaner on contact with human hair, would be as follows in an aqueous solution:
Another reaction that may occur, reaction 2, would take place as follows in an aqueous solution:
Compared to the organic compound produced in Reaction 2, an aldehyde __________.
Which of the following functional groups would most likely act as an acid?
Understanding the function of the sodium-potassium pump, which of the following residues might possibly line the channel?
Your lab isolates a compound with the formula 
Among the most important pH buffer systems in humans is the bicarbonate buffer, which keeps the blood at a remarkably precise 7.42 pH. The bicarbonate buffer system uses a series of important compounds and enzymes to make the system function. Figure 1 depicts the key reactions that take place.

The activity of this buffer system is mainly controlled by the renal and respiratory systems. The renal system excretes bicarbonate in the urine, while the respiratory system “blows off” carbon dioxide as needed. By balancing these two systems as needed, blood pH is maintained in such a narrow range.
The deprotonation of carbonic acid is favored by __________.