Units - High School Chemistry
Card 0 of 20
What is the concentration of Ca in a solution of 1 mol CaCl2 in 1 L of distilled water? (M = molarity, m= molality)
What is the concentration of Ca in a solution of 1 mol CaCl2 in 1 L of distilled water? (M = molarity, m= molality)
The definition of molality is moles of solute in 1 kg of the solvent, whereas molarity is the number of moles of solute per 1 L of solutioin. Since 1 mol of CaCl2 is added to 1 L of water, this means that the volume of the final solution is greater than 1 L. Thus, molality is the more accurate concentration determinant, since the solution is probably close to 1 L.
The definition of molality is moles of solute in 1 kg of the solvent, whereas molarity is the number of moles of solute per 1 L of solutioin. Since 1 mol of CaCl2 is added to 1 L of water, this means that the volume of the final solution is greater than 1 L. Thus, molality is the more accurate concentration determinant, since the solution is probably close to 1 L.
Compare your answer with the correct one above
Which of the following is equivalent to molarity?
Which of the following is equivalent to molarity?
Molarity, molality, and normality are the three principle ways to measure concentration. Molarity is a measure of moles of solute per liter of solution. Molality is a measure of moles of solute per kilogram of solvent. Normality expressly relates to acids and bases, and is the measure of moles of solute divided by the number of hydrogen equivalents per mole, all divided by liters of solution. Normality is also referred to as "equivalents (of acid) per liter."
Molarity, molality, and normality are the three principle ways to measure concentration. Molarity is a measure of moles of solute per liter of solution. Molality is a measure of moles of solute per kilogram of solvent. Normality expressly relates to acids and bases, and is the measure of moles of solute divided by the number of hydrogen equivalents per mole, all divided by liters of solution. Normality is also referred to as "equivalents (of acid) per liter."
Compare your answer with the correct one above
How many moles of  are in
are in  of a
of a  solution?
solution?
How many moles of 


Molarity is defined as moles of solute divided into liters of solution. We can set up the equation as follows:

Set the left side of the equation over  and solve as a proportion.
and solve as a proportion.

Cross multiply.

Remember molarity is defined as moles of solute per liter of solution.

Substiute.

Liters cancel out.

Solve for the number of moles. This gives us  of solute in a
of solute in a  ,
,  solution of
solution of  .
.
Molarity is defined as moles of solute divided into liters of solution. We can set up the equation as follows:
Set the left side of the equation over 
Cross multiply.
Remember molarity is defined as moles of solute per liter of solution.
Substiute.
Liters cancel out.
Solve for the number of moles. This gives us 



Compare your answer with the correct one above
Which of the following units is common for measuring concentration?
Which of the following units is common for measuring concentration?
 is the dimensional breakdown for the commonly used unit
is the dimensional breakdown for the commonly used unit  , or molarity, for concentration.
, or molarity, for concentration.
While the other units might be seen in chemistry, they are used for other topics.
Millimeters of mercury (mmHg) and atmospheres (atm) are seen in regards to pressure.
 is the dimensional breakdown for density. And
is the dimensional breakdown for density. And  is the dimensional breakdown for a Newton, also written as N. This is associated with force.
is the dimensional breakdown for a Newton, also written as N. This is associated with force.


While the other units might be seen in chemistry, they are used for other topics.
Millimeters of mercury (mmHg) and atmospheres (atm) are seen in regards to pressure.


Compare your answer with the correct one above
How many atoms are in 1 mole of H2?
How many atoms are in 1 mole of H2?
This question requires an understanding of what avogadro's number actually represents. Avogadro's number, 6.022 * 1023 is the number of things in one mole. The question indicates that there is 1 mole of H2. Thus there are 6.022 * 1023 molecules of H2. However the question is asking for the amount of atoms in 1 mole of H2. Thus we must consider the makeup of an H2 molecule, where we see that it is a diatomic molecule. Thus we must multiply 6.022 * 1023 by 2 to calculate the number of individual atoms present in 1 mole of H2. We find our answer to be 1.2044 * 1024.
This question requires an understanding of what avogadro's number actually represents. Avogadro's number, 6.022 * 1023 is the number of things in one mole. The question indicates that there is 1 mole of H2. Thus there are 6.022 * 1023 molecules of H2. However the question is asking for the amount of atoms in 1 mole of H2. Thus we must consider the makeup of an H2 molecule, where we see that it is a diatomic molecule. Thus we must multiply 6.022 * 1023 by 2 to calculate the number of individual atoms present in 1 mole of H2. We find our answer to be 1.2044 * 1024.
Compare your answer with the correct one above
A chemist has  of
of  .
.
How many molecules of  does she have?
does she have?
A chemist has 

How many molecules of 
Compare your answer with the correct one above
What is the mass of  particles of
particles of  ?
?
What is the mass of 

Compare your answer with the correct one above
How many atoms are in  of calcium?
of calcium?
How many atoms are in 
In order to determine how many atoms are in this sample, we need to convert this sample into moles. Calcium has a molar mass of  grams per mole.
grams per mole.

Avogadro's number tells us that there  atoms in one mole of any element. We can use this conversion to find the total number of atoms in the sample.
atoms in one mole of any element. We can use this conversion to find the total number of atoms in the sample.

In order to determine how many atoms are in this sample, we need to convert this sample into moles. Calcium has a molar mass of 
Avogadro's number tells us that there 
Compare your answer with the correct one above
How many hydrogen atoms are in 46.3 g of ethanol,  ?
?
How many hydrogen atoms are in 46.3 g of ethanol, 
To determine the number of hydrogen atoms, divide the mass of ethanol by its molar mass to get moles of ethanol.


Multiply this by six atoms of hydrogen per molecule of ethanol and by Avogadro's number to get the number of hydrogen atoms.


To determine the number of hydrogen atoms, divide the mass of ethanol by its molar mass to get moles of ethanol.
Multiply this by six atoms of hydrogen per molecule of ethanol and by Avogadro's number to get the number of hydrogen atoms.
Compare your answer with the correct one above
How many hydrogen atoms are present in 500 mL of water at room temperature?

How many hydrogen atoms are present in 500 mL of water at room temperature?
Use the density of water, the molar mass of water, and Avogadro's number to calculate the number of molecules of water.
We have 500mL of water. Use the density to convert this to grams; then use the molar mass of water to convert this to moles.

There are two moles of hydrogen atoms per one mole of water.

Finally, multiply by Avogadro's number.

Use the density of water, the molar mass of water, and Avogadro's number to calculate the number of molecules of water.
We have 500mL of water. Use the density to convert this to grams; then use the molar mass of water to convert this to moles.
There are two moles of hydrogen atoms per one mole of water.
Finally, multiply by Avogadro's number.
Compare your answer with the correct one above
How many molecules are in  ?
?
How many molecules are in 
According to Avogadro's law, each mole of a compound contains  . Use dimensional analysis to find the number of molecules in
. Use dimensional analysis to find the number of molecules in  .
.

According to Avogadro's law, each mole of a compound contains 

Compare your answer with the correct one above
How many atoms are there in 47.5g of boron?
How many atoms are there in 47.5g of boron?
To do this problem we have to first convert grams to moles, then moles to atoms using Avogadro's number:

To do this problem we have to first convert grams to moles, then moles to atoms using Avogadro's number:
Compare your answer with the correct one above
How many moles of carbon are in a sample of  atoms?
atoms?
How many moles of carbon are in a sample of 
To solve, we need to convert atoms to moles using Avogadro's number:

To solve, we need to convert atoms to moles using Avogadro's number:
Compare your answer with the correct one above
How many atoms of sodium are in three moles of  ?
?
How many atoms of sodium are in three moles of 
To answer this question, we have to find the number of moles of sodium in this compound. Note that there are two sodium atoms per molecule. Then, multiply the total moles of sodium by Avogadro's number.

To answer this question, we have to find the number of moles of sodium in this compound. Note that there are two sodium atoms per molecule. Then, multiply the total moles of sodium by Avogadro's number.
Compare your answer with the correct one above
You have a neutral balloon. If you were to add 21,000 electrons to it, what would its net charge be?
 = charge of one electron
= charge of one electron
You have a neutral balloon. If you were to add 21,000 electrons to it, what would its net charge be?

The elemental charge is the magnitude of charge, in Coulombs, that each electron or proton has. Because electrons have a negative charge, don't forget to add a negative sign into the equation.



When you convert the answer to microcoulombs, the answer is  :
:

The elemental charge is the magnitude of charge, in Coulombs, that each electron or proton has. Because electrons have a negative charge, don't forget to add a negative sign into the equation.
When you convert the answer to microcoulombs, the answer is 
Compare your answer with the correct one above
Determine the number of sodium atoms in a  block of sodium chloride.
block of sodium chloride.
Determine the number of sodium atoms in a 
Use dimensional analysis:

Use dimensional analysis:
Compare your answer with the correct one above
A container has  molecules of gas in it. How many moles of the gas are in the container?
molecules of gas in it. How many moles of the gas are in the container?
A container has 
Avogadro's number tells us how many molecules of gas are in one mole of the container. We are essentially doing a unit conversion from "number of molecules" to moles -

Avogadro's number tells us how many molecules of gas are in one mole of the container. We are essentially doing a unit conversion from "number of molecules" to moles -
Compare your answer with the correct one above
Consider the following four samples:
 of potassium
of potassium
 of lithium
of lithium
 of magnesium
of magnesium
 of chlorine gas
of chlorine gas
Which of the given samples contains the most atoms?
Consider the following four samples:




Which of the given samples contains the most atoms?
It is important to note that the mass of a sample does not tell you the amount of atoms in the sample. The number of atoms in a sample is dependent on the number moles in a sample, given by Avogadro's number. Here is the number of moles for each sample:




Remember that chlorine is a diatomic mass, so each molecules contains two atoms. This doubles the molar mass for the conversion.
The sample with the greatest number of moles will also contain the most atoms. In this case, the sample of lithium results in the largest number of moles and, thus, the greatest number of atoms.
It is important to note that the mass of a sample does not tell you the amount of atoms in the sample. The number of atoms in a sample is dependent on the number moles in a sample, given by Avogadro's number. Here is the number of moles for each sample:
Remember that chlorine is a diatomic mass, so each molecules contains two atoms. This doubles the molar mass for the conversion.
The sample with the greatest number of moles will also contain the most atoms. In this case, the sample of lithium results in the largest number of moles and, thus, the greatest number of atoms.
Compare your answer with the correct one above
Convert the following amount from grams (g) to moles (m)
How many moles is  of
of  ?
?
Convert the following amount from grams (g) to moles (m)
How many moles is 

Use the periodic table to calculate the molecular weight of sodium hydroxide.

Next, use dimensional analysis to find the number of moles.

Use the periodic table to calculate the molecular weight of sodium hydroxide.
Next, use dimensional analysis to find the number of moles.
Compare your answer with the correct one above

Consider the reaction above. If you start with  of potassium bromide, how many moles of bromine are produced? How many molecules is this equal to?
of potassium bromide, how many moles of bromine are produced? How many molecules is this equal to?
Consider the reaction above. If you start with 
In the chemical equation, the ratio of potassium bromide to bromine is 2:1, so for every 2 moles of  , 1 mole of
, 1 mole of 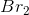 is produced. Therefore, if we start with 4 moles of
is produced. Therefore, if we start with 4 moles of  , we get 2 moles of
, we get 2 moles of  . The number of molecules is equal to the number of moles times Avogadro's Number. Since we've determined the number of moles to be 2, the number of molecules is:
. The number of molecules is equal to the number of moles times Avogadro's Number. Since we've determined the number of moles to be 2, the number of molecules is:
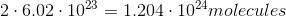
In the chemical equation, the ratio of potassium bromide to bromine is 2:1, so for every 2 moles of 


Compare your answer with the correct one above

