Using Charles's Law
Practice Questions
High School Chemistry › Using Charles's Law
Which law is the following formula?
A balloon filled with room temperature air (


A canister of gas has a volume of 


A gas occupies a volume of 


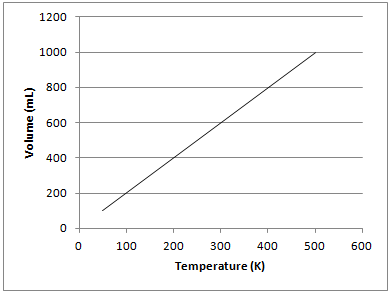
The graph depicted below represents which of the gas laws?




If pressure is kept constant, what is the final volume of the gas if the temperature of the container is increased to