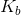Using Base Dissociation Constant (Kb)
Practice Questions
High School Chemistry › Using Base Dissociation Constant (Kb)
Questions
1
1
A 1M solution of a monoprotic acid has a pH of 4.6. What is the 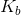
High School Chemistry › Using Base Dissociation Constant (Kb)
A 1M solution of a monoprotic acid has a pH of 4.6. What is the