Titrations - GRE Subject Test: Chemistry
Card 0 of 11
A weak acid is slowly titrated with a strong base. Where on the titration curve would the solution be the most well-buffered?
A weak acid is slowly titrated with a strong base. Where on the titration curve would the solution be the most well-buffered?
At the half equivalence point, the concentration of acid in the solution is equal to the concentration of the conjugate base in solution. At this point, the graph shows a line that is near horizontal. This means that base or acid could be added and the pH of the solution would change very slowly.
Remember that pH is on the y-axis of the titration curve; thus a near-horizontal line will signify a region where pH is most stable. At the half equivalence point, pH = pKa.
At the half equivalence point, the concentration of acid in the solution is equal to the concentration of the conjugate base in solution. At this point, the graph shows a line that is near horizontal. This means that base or acid could be added and the pH of the solution would change very slowly.
Remember that pH is on the y-axis of the titration curve; thus a near-horizontal line will signify a region where pH is most stable. At the half equivalence point, pH = pKa.
Compare your answer with the correct one above
Which of the following pH values is an acceptable equivalence point for a weak base being titrated by a strong acid?
Which of the following pH values is an acceptable equivalence point for a weak base being titrated by a strong acid?
The equivalence point is the point during a titration when there are equal equivalents of acid and base in the solution. Since a strong acid will have more effect on the pH than the same amount of a weak base, we predict that the solution's pH will be acidic at the equivalence point. 5.2 and 1.3 are both acidic, but 1.3 is remarkably acidic considering that there is an equal amount of base in the solution. As a result, 5.2 is a more appropriate answer.
The equivalence point for a strong acid and strong base will be 7.0. When one of the compounds is weak, however, it will dissociate less than its strong counterpart. In our case, the base will dissociate less than the acid. The acid, thus, contributes more to the pH character of the solution.
The equivalence point is the point during a titration when there are equal equivalents of acid and base in the solution. Since a strong acid will have more effect on the pH than the same amount of a weak base, we predict that the solution's pH will be acidic at the equivalence point. 5.2 and 1.3 are both acidic, but 1.3 is remarkably acidic considering that there is an equal amount of base in the solution. As a result, 5.2 is a more appropriate answer.
The equivalence point for a strong acid and strong base will be 7.0. When one of the compounds is weak, however, it will dissociate less than its strong counterpart. In our case, the base will dissociate less than the acid. The acid, thus, contributes more to the pH character of the solution.
Compare your answer with the correct one above
Which of the following curves represents the titration of a strong base by a strong acid?
Which of the following curves represents the titration of a strong base by a strong acid?
Since we are adding acid to a base, the pH must decrease. The initial base will have a high pH, while the final acid will have a low pH; the right of the curve must be lower than the left. In addition, the pH does not change very rapidly until the equivalence point is reached, hence the curve must show little initial change followed by a rapid change.
Since we are adding acid to a base, the pH must decrease. The initial base will have a high pH, while the final acid will have a low pH; the right of the curve must be lower than the left. In addition, the pH does not change very rapidly until the equivalence point is reached, hence the curve must show little initial change followed by a rapid change.
Compare your answer with the correct one above
Which of the following is an indication of when a reaction has reached the equivalence point.
Which of the following is an indication of when a reaction has reached the equivalence point.
The equivalence point is the point in a titration at which the number of moles of titrant equals the number of moles of analyte. The endpoint in a titration is when the color of the reaction changes to that of the desired (often neutral) pH. However this is not the same as the equivalence point, which is a stoichiometric value. Indicators may change colors several times throughout a neutralization reaction, thus this is not an accurate attribute of the reaction to use as the equivalence point. Furthermore, the concentrations of titrant and the analyte are rarely equivalent, and there could be differing numbers of ionizable groups on each species.
The equivalence point is the point in a titration at which the number of moles of titrant equals the number of moles of analyte. The endpoint in a titration is when the color of the reaction changes to that of the desired (often neutral) pH. However this is not the same as the equivalence point, which is a stoichiometric value. Indicators may change colors several times throughout a neutralization reaction, thus this is not an accurate attribute of the reaction to use as the equivalence point. Furthermore, the concentrations of titrant and the analyte are rarely equivalent, and there could be differing numbers of ionizable groups on each species.
Compare your answer with the correct one above

Considering the given neutralization reaction, what is the concentration of the sodium chloride solution if it takes 20.00mL of 0.045M  to neutralize 20mL of the
to neutralize 20mL of the  solution?
solution?
Considering the given neutralization reaction, what is the concentration of the sodium chloride solution if it takes 20.00mL of 0.045M 

Based on the equation given, we know the that  reacts with
reacts with  in a 1:1 mole ratio. We can use the molar concentration given as a conversion factor to determine the number of moles of
in a 1:1 mole ratio. We can use the molar concentration given as a conversion factor to determine the number of moles of  used which will be equal to the number of moles of
used which will be equal to the number of moles of  used.
used.

To determine the molar concentration of  used, we can simply use the following equation:
used, we can simply use the following equation:

We have determined that the concentration of  used is equal to the concentration of
used is equal to the concentration of  used.
used.
Based on the equation given, we know the that 



To determine the molar concentration of 
We have determined that the concentration of 

Compare your answer with the correct one above

Considering the given chemical reaction, determine the number of moles of  in a 20mL solution if it takes 19.00mL of a 0.0500M
in a 20mL solution if it takes 19.00mL of a 0.0500M  solution to reach the endpoint of a titration.
solution to reach the endpoint of a titration.
Considering the given chemical reaction, determine the number of moles of 

 and
and  react in a 1:1 mole ratio. Therefore the number of moles of
react in a 1:1 mole ratio. Therefore the number of moles of  at the end point of the reaction equals to the number of moles of
at the end point of the reaction equals to the number of moles of  . We can use the concentration as a conversion factor to determine the number of moles reacted.
. We can use the concentration as a conversion factor to determine the number of moles reacted.





Compare your answer with the correct one above

Considering the given chemical reaction, determine the concentration of  in a 40.00mL solution if it takes 25.00mL of a 0.0350M
in a 40.00mL solution if it takes 25.00mL of a 0.0350M  solution to reach the endpoint of a titration.
solution to reach the endpoint of a titration.
Considering the given chemical reaction, determine the concentration of 

 and
and  react in a 1:1 mole ratio. Therefore the number of moles of
react in a 1:1 mole ratio. Therefore the number of moles of  at the end point of the reaction equals to the number of moles of
at the end point of the reaction equals to the number of moles of  .
.






Compare your answer with the correct one above

A vinegar sample was determined by a titration with  to have
to have  moles of
moles of  in a 2.00 grams of vinegar. What would be the percent mass of this vinegar solution?
in a 2.00 grams of vinegar. What would be the percent mass of this vinegar solution?
A vinegar sample was determined by a titration with 


We must first calculate the molecular weight of  :
:

We can calculate the grams of  using the molecular weight calculated as a conversion factor:
using the molecular weight calculated as a conversion factor:

Finally, we can calculate the percent mass using the total grams of vinegar solution:

We must first calculate the molecular weight of 
We can calculate the grams of 
Finally, we can calculate the percent mass using the total grams of vinegar solution:
Compare your answer with the correct one above

Based on the pH titration curve provided, how many acidic protons are in this acid?

Based on the pH titration curve provided, how many acidic protons are in this acid?
The equivalence point is the point in a titration at which the number of moles of titrant species equals the number of moles of analyte. For a polyprotic acid there are multiple equivalence points because there are more than one acidic proton in one molecule of the acid. A titration curve has as many equivalence points as the number of protons that may be neutralized by the interaction with a base. In this titration curve there are two equivalence points. The equivalence points occur when the graph has a very steep slope, and involve very large changes in pH with additions of small amounts of base. For this graph, the equivalence points occur at about pH 3.5 and 10.
The equivalence point is the point in a titration at which the number of moles of titrant species equals the number of moles of analyte. For a polyprotic acid there are multiple equivalence points because there are more than one acidic proton in one molecule of the acid. A titration curve has as many equivalence points as the number of protons that may be neutralized by the interaction with a base. In this titration curve there are two equivalence points. The equivalence points occur when the graph has a very steep slope, and involve very large changes in pH with additions of small amounts of base. For this graph, the equivalence points occur at about pH 3.5 and 10.
Compare your answer with the correct one above
What volume in liters of 
 is needed in the titration of
is needed in the titration of  of a
of a 
 (acetic acid) solution to reach the equivalence point?
(acetic acid) solution to reach the equivalence point?
What volume in liters of 




The equivalence point in a titration is the point in which the number of moles of titrant equals to the number of moles of analyte:

We need to determine the number of moles of acetic acid we are dealing with:


Therefore, at the equivalence point there are also 0.0075 moles of  in the solution. In order to determine the number of moles of
in the solution. In order to determine the number of moles of  in solution, we must use the concentration of
in solution, we must use the concentration of  as a conversion factor to determine the volume added to the acetic acid solution.
as a conversion factor to determine the volume added to the acetic acid solution.

The equivalence point in a titration is the point in which the number of moles of titrant equals to the number of moles of analyte:
We need to determine the number of moles of acetic acid we are dealing with:
Therefore, at the equivalence point there are also 0.0075 moles of 


Compare your answer with the correct one above
A 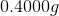 sample of a monoprotic acid was titrated against a
sample of a monoprotic acid was titrated against a  solution of
solution of  . If the end point was reached after adding
. If the end point was reached after adding  , what is the molar mass of the weak acid?
, what is the molar mass of the weak acid?
A 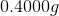



A pH indicator added to a solution causes a color change which is an indication that a reaction has reached the equivalence point. The equivalence point is when the number of moles of titrant equals the number of moles of analyte. The end point is an approximation of this in a titration.
At the equivalence point:

The number of moles of NaOH at equivalence point is calculated as follows:


Assuming we were trying to convert the number of grams of the monoprotic acid to moles, the equation would be set up as follows:

Let's plug the values we have into this equation and give the molar mass a value of  :
:

Rearranging this equation to solve for the molar mass  gives:
gives:

A pH indicator added to a solution causes a color change which is an indication that a reaction has reached the equivalence point. The equivalence point is when the number of moles of titrant equals the number of moles of analyte. The end point is an approximation of this in a titration.
At the equivalence point:
The number of moles of NaOH at equivalence point is calculated as follows:
Assuming we were trying to convert the number of grams of the monoprotic acid to moles, the equation would be set up as follows:
Let's plug the values we have into this equation and give the molar mass a value of 
Rearranging this equation to solve for the molar mass 
Compare your answer with the correct one above