Physical Chemistry - GRE Subject Test: Chemistry
Card 0 of 20
Two moles of sodium chloride (NaCl) are added to 1kg of a mystery solvent. The addition of the NaCl caused an increase of 6K to the solvent's boiling point.
Based on this information, what is the boiling constant for the solvent?
Two moles of sodium chloride (NaCl) are added to 1kg of a mystery solvent. The addition of the NaCl caused an increase of 6K to the solvent's boiling point.
Based on this information, what is the boiling constant for the solvent?
In order to solve this problem, we can use the boiling point elevation equation:  .
.
We know the temperature change, we can compute molality from the given information, and we know the van't Hoff factor (expected to be 2 in this scenario due to NaCl becoming 2 ions in solution). We can calculate the boiling point constant for the solvent.




In order to solve this problem, we can use the boiling point elevation equation: 
We know the temperature change, we can compute molality from the given information, and we know the van't Hoff factor (expected to be 2 in this scenario due to NaCl becoming 2 ions in solution). We can calculate the boiling point constant for the solvent.
Compare your answer with the correct one above
What is the boiling point of a solution composed of three liters of water and 50 grams of sodium chloride?


The molar mass of sodium chloride is  .
.
What is the boiling point of a solution composed of three liters of water and 50 grams of sodium chloride?
The molar mass of sodium chloride is 
We can use the boiling point elevation equation in order to determine the new boiling point once the salt has been added:

Since sodium chloride will form two ions for each molecule in solution, the value for the van't Hoff factor will be 2. In addition, the mass of the water in the solution will be 3 kilograms, which can be determined by using the density of water.


Since water boils at 100 degrees Celsius, this means that the final boiling point of the solution is 100.29 degrees Celsius.
We can use the boiling point elevation equation in order to determine the new boiling point once the salt has been added:
Since sodium chloride will form two ions for each molecule in solution, the value for the van't Hoff factor will be 2. In addition, the mass of the water in the solution will be 3 kilograms, which can be determined by using the density of water.
Since water boils at 100 degrees Celsius, this means that the final boiling point of the solution is 100.29 degrees Celsius.
Compare your answer with the correct one above
The equation for the change in Gibbs free energy is given below.

ΔH = change in enthalpy
ΔS = change in entropy
Which of the following scenarios guarantees a nonspontaneous reaction?
The equation for the change in Gibbs free energy is given below.
ΔH = change in enthalpy
ΔS = change in entropy
Which of the following scenarios guarantees a nonspontaneous reaction?
A positive value for ΔG (Gibbs free energy) will guarantee a nonspontaneous reaction. When ΔH (enthalpy) is postive and ΔS (entropy) is negative, the change in Gibbs free energy must be positive and, therefore, nonspontaneous.


Because T (temperature) will always have a positive value, a negative entropy and positive enthalpy will always result in a positive Gibbs free energy.
A positive value for ΔG (Gibbs free energy) will guarantee a nonspontaneous reaction. When ΔH (enthalpy) is postive and ΔS (entropy) is negative, the change in Gibbs free energy must be positive and, therefore, nonspontaneous.
Because T (temperature) will always have a positive value, a negative entropy and positive enthalpy will always result in a positive Gibbs free energy.
Compare your answer with the correct one above
The equation for the change in Gibbs free energy is given below.

ΔH = change in enthalpy
ΔS = change in entropy
In which of the scenarios will the reaction be spontaneous?
The equation for the change in Gibbs free energy is given below.
ΔH = change in enthalpy
ΔS = change in entropy
In which of the scenarios will the reaction be spontaneous?
All of the following scenarios would lead to spontaneous reaction, since each scenario would result in a negative Gibbs free energy (-ΔG).

Negative enthalpy, positive entropy:

Positive enthalpy and entropy with high temperature:

Negative enthalpy and entropy with low temperature:

All of the following scenarios would lead to spontaneous reaction, since each scenario would result in a negative Gibbs free energy (-ΔG).
Negative enthalpy, positive entropy:
Positive enthalpy and entropy with high temperature:
Negative enthalpy and entropy with low temperature:
Compare your answer with the correct one above
Which of the following scenarios describes a reaction in equilibrium?
Which of the following scenarios describes a reaction in equilibrium?
According to the Gibbs free energy equation, a system is at equilibrium when  is equal to 0.
is equal to 0.
Since  , a system is in equilibrium when
, a system is in equilibrium when  . A negative Gibbs free energy means that the reaction will be spontaneous. At equilibrium, the forward reaction rate equals the reverse reaction rate, though the net rate is zero.
. A negative Gibbs free energy means that the reaction will be spontaneous. At equilibrium, the forward reaction rate equals the reverse reaction rate, though the net rate is zero.
According to the Gibbs free energy equation, a system is at equilibrium when 
Since 

Compare your answer with the correct one above
When the enthalpy of a reaction has a value of  , which word is used to describe the reaction?
, which word is used to describe the reaction?
When the enthalpy of a reaction has a value of 
When the enthalpy of a reaction is negative, it means that heat has been released from the system to the surroundings. This release of energy is referred to as an exothermic reaction.
Endothermic reactions have a net heat flow into the system. Exergonic and endergonic are terms used to describe the change in free energy for a given system.
When the enthalpy of a reaction is negative, it means that heat has been released from the system to the surroundings. This release of energy is referred to as an exothermic reaction.
Endothermic reactions have a net heat flow into the system. Exergonic and endergonic are terms used to describe the change in free energy for a given system.
Compare your answer with the correct one above
For any given chemical reaction, one can draw an energy diagram. Energy diagrams depict the energy levels of the different steps in a reaction, while also indicating the net change in energy and giving clues to relative reaction rate.
Below, a reaction diagram is shown for a reaction that a scientist is studying in a lab. A student began the reaction the evening before, but the scientist is unsure as to the type of the reaction. He cannot find the student’s notes, except for the reaction diagram below.

Upon further review, the scientist realizes that the reaction in question involved formation of a carbocation that quickly reacted again to form stable products. At which point would we most likely find this carbocation in the above diagram?
For any given chemical reaction, one can draw an energy diagram. Energy diagrams depict the energy levels of the different steps in a reaction, while also indicating the net change in energy and giving clues to relative reaction rate.
Below, a reaction diagram is shown for a reaction that a scientist is studying in a lab. A student began the reaction the evening before, but the scientist is unsure as to the type of the reaction. He cannot find the student’s notes, except for the reaction diagram below.

Upon further review, the scientist realizes that the reaction in question involved formation of a carbocation that quickly reacted again to form stable products. At which point would we most likely find this carbocation in the above diagram?
Point 3 is where you would expect to find a relatively stable intermediate. An intermediate is more stable than a transition state, but not as stable as the original reactants and final products. Stability is inversely proportional to energy, thus we are looking for the point that is between the highest and lowest energies in the reaction. By this logic, point 1 is the reactants, 2 and 4 are transition states, 3 is a stable intermediate, and 5 is the products.
Point 3 is where you would expect to find a relatively stable intermediate. An intermediate is more stable than a transition state, but not as stable as the original reactants and final products. Stability is inversely proportional to energy, thus we are looking for the point that is between the highest and lowest energies in the reaction. By this logic, point 1 is the reactants, 2 and 4 are transition states, 3 is a stable intermediate, and 5 is the products.
Compare your answer with the correct one above



Given the enthalpies of formation, what is the enthalpy of combustion of octane in the reaction:

Given the enthalpies of formation, what is the enthalpy of combustion of octane in the reaction:
The equation for enthalpy of reaction is:

Given our chemical reaction and the enthalpies of formation, we can find the enthalpy of reaction.

First, find the total enthalpy for the products.


Then, find the total enthalpy for the reactants.


Since the oxygen is elemental, its heat of formation is zero.
Return to the original equation to calculate the final enthalpy of reaction.

The equation for enthalpy of reaction is:
Given our chemical reaction and the enthalpies of formation, we can find the enthalpy of reaction.
First, find the total enthalpy for the products.
Then, find the total enthalpy for the reactants.
Since the oxygen is elemental, its heat of formation is zero.
Return to the original equation to calculate the final enthalpy of reaction.
Compare your answer with the correct one above
Propane gas combusts according the following chemical equation:

Given the following standard enthalpy values, what is the enthalpy of the reaction for the combustion of one mole of propane?




Propane gas combusts according the following chemical equation:
Given the following standard enthalpy values, what is the enthalpy of the reaction for the combustion of one mole of propane?
Given the molar enthalpy values for reactants and products, we can solve for the enthalpy of the reaction using the equation:

Keep in mind that the molar enthalpy values must be multiplied by the coefficients that are present in the balanced reaction:


Oxygen is omitted because its enthalpy value is zero.


Given the molar enthalpy values for reactants and products, we can solve for the enthalpy of the reaction using the equation:
Keep in mind that the molar enthalpy values must be multiplied by the coefficients that are present in the balanced reaction:
Oxygen is omitted because its enthalpy value is zero.
Compare your answer with the correct one above
Which is not characteristic of an endothermic reaction?
Which is not characteristic of an endothermic reaction?
An endothermic reaction absorbs energy in the form of heat. An endothermic reaction involves breaking of chemical bonds because it involves the absorption of energy. Endothermic reactions tend to feel cold because it is taking heat away from your skin. Since endothermic reactions involve absorbing energy, often in the form of heat, the change in enthalpy is positive.
Therefore, the answer is "releasing of energy to surroundings" which is not a characteristic of an endothermic process. Instead, it is a characteristic of an exothermic reaction which is the direct opposite of an endothermic reaction.
An endothermic reaction absorbs energy in the form of heat. An endothermic reaction involves breaking of chemical bonds because it involves the absorption of energy. Endothermic reactions tend to feel cold because it is taking heat away from your skin. Since endothermic reactions involve absorbing energy, often in the form of heat, the change in enthalpy is positive.
Therefore, the answer is "releasing of energy to surroundings" which is not a characteristic of an endothermic process. Instead, it is a characteristic of an exothermic reaction which is the direct opposite of an endothermic reaction.
Compare your answer with the correct one above
Which of the following reactions has a positive value for change in entropy  ?
?
Which of the following reactions has a positive value for change in entropy 
Entropy is a measure of the disorder of a system. In other words, when the components of a system become more uniform and spread out, the entropy of the system has increased. For example, when a liquid becomes gaseous, the molecules separate from one another, increasing the disorder of the system. As a result, the evaporation of water results in an increase in entropy.
All other options result in a more ordered system, which decreases entropy.
Entropy is a measure of the disorder of a system. In other words, when the components of a system become more uniform and spread out, the entropy of the system has increased. For example, when a liquid becomes gaseous, the molecules separate from one another, increasing the disorder of the system. As a result, the evaporation of water results in an increase in entropy.
All other options result in a more ordered system, which decreases entropy.
Compare your answer with the correct one above
What is the freezing point of a 2M solution of  in water?
in water?

What is the freezing point of a 2M solution of 

First, we need to calculate the molality because that is what we use in our equation for freezing point depression. We can get that from the molarity without knowing exactly how many liters or grams we have. We just have to know what we have one mole per liter. The weight of water is one kilogram per liter, so this allows us to make this conversion.

The molality is 2m. The van't Hoff factor is 3, as we get one calcium ion and two chloride ions per molecule during dissociation.

We can now plug the values into the equation for freezing point depression.


This gives us our depression of  . The normal freezing point of pure water is
. The normal freezing point of pure water is  , which means our new freezing point is
, which means our new freezing point is  .
.
First, we need to calculate the molality because that is what we use in our equation for freezing point depression. We can get that from the molarity without knowing exactly how many liters or grams we have. We just have to know what we have one mole per liter. The weight of water is one kilogram per liter, so this allows us to make this conversion.
The molality is 2m. The van't Hoff factor is 3, as we get one calcium ion and two chloride ions per molecule during dissociation.
We can now plug the values into the equation for freezing point depression.
This gives us our depression of 


Compare your answer with the correct one above
How much sodium chloride has been added to four liters of water if the freezing point of the solution is  ?
?


Sodium chloride has a molar mass of  .
.
How much sodium chloride has been added to four liters of water if the freezing point of the solution is 
Sodium chloride has a molar mass of 
We can determine how much sodium chloride was added to the water using the freezing point depression equation.

The normal freezing point of wtaer is 0 degrees Celsius, so we know that the temperature of the solution has changed by 2.5 degrees. Since sodium chloride will generate two ions per molecule in solution, the van't Hoff factor will be 2. Based on the density of water, we can determine that 4 liters of water weighs 4 kilograms.


We can determine how much sodium chloride was added to the water using the freezing point depression equation.
The normal freezing point of wtaer is 0 degrees Celsius, so we know that the temperature of the solution has changed by 2.5 degrees. Since sodium chloride will generate two ions per molecule in solution, the van't Hoff factor will be 2. Based on the density of water, we can determine that 4 liters of water weighs 4 kilograms.
Compare your answer with the correct one above
A gas sample is contained in a 4L vessel at a pressure of 3atm. Assuming all other conditions are kept constant, what is the new pressure in the vessel if the volume is reduced to 1.5L?
A gas sample is contained in a 4L vessel at a pressure of 3atm. Assuming all other conditions are kept constant, what is the new pressure in the vessel if the volume is reduced to 1.5L?
According to Boyle's law, pressure and volume are inversely proprotional to each other. This is represented by the equation:

In other words, as volume decreases in a vessel, the pressure will increase, and vice versa. Using the given conditions, we can solve for the final pressure in the vessel:


According to Boyle's law, pressure and volume are inversely proprotional to each other. This is represented by the equation:
In other words, as volume decreases in a vessel, the pressure will increase, and vice versa. Using the given conditions, we can solve for the final pressure in the vessel:
Compare your answer with the correct one above
An unknown amount of neon gas is contained in a 3.00L vessel. At a temperature of  , the gas exerts a pressure of 4.00atm.
, the gas exerts a pressure of 4.00atm.

Neon gas has a molar mass of  .
.
Based on these conditions, what is the mass of neon gas in the vessel?
An unknown amount of neon gas is contained in a 3.00L vessel. At a temperature of 
Neon gas has a molar mass of 
Based on these conditions, what is the mass of neon gas in the vessel?
This question deals with the amount of gas present in a vessel for only one set of conditions. This makes the ideal gas law a suitable equation to use in order to determine the amount of gas in the vessel. The ideal gas law is written as:

Using this equation, we can solve for the molar quantity of gas in the vessel:



Knowing this, we can now solve for the mass of the gas in the vessel by multiplying this molar amount by the molar mass:



This question deals with the amount of gas present in a vessel for only one set of conditions. This makes the ideal gas law a suitable equation to use in order to determine the amount of gas in the vessel. The ideal gas law is written as:
Using this equation, we can solve for the molar quantity of gas in the vessel:
Knowing this, we can now solve for the mass of the gas in the vessel by multiplying this molar amount by the molar mass:
Compare your answer with the correct one above
Which gas follows the exact definition of the ideal gas law?
Which gas follows the exact definition of the ideal gas law?
Though the ideal gas law gives a nearly close to real approximation of numbers, it oversimplifies its description of gases. No real gas follows the exact definition of the ideal gas law and is very complex because there are intermolecular forces that must be considered. An ideal gas described as a point mass in which the particles are so small that its volume is negligible. However, real gases have real volume. Also, ideal gases are considered elastic, having no attractive and repulsive forces with no energy transfer during collisions. Real gases actually collide and are non-elastic. Note that gases approach ideal behavior as their temperature increases and their pressure decreases.
Though the ideal gas law gives a nearly close to real approximation of numbers, it oversimplifies its description of gases. No real gas follows the exact definition of the ideal gas law and is very complex because there are intermolecular forces that must be considered. An ideal gas described as a point mass in which the particles are so small that its volume is negligible. However, real gases have real volume. Also, ideal gases are considered elastic, having no attractive and repulsive forces with no energy transfer during collisions. Real gases actually collide and are non-elastic. Note that gases approach ideal behavior as their temperature increases and their pressure decreases.
Compare your answer with the correct one above
At  , a reaction has a Gibb's free energy change of
, a reaction has a Gibb's free energy change of  . If the enthalpy change of the reaction is
. If the enthalpy change of the reaction is  , what is the entropy change of the reaction?
, what is the entropy change of the reaction?
At 


We can relate enthalpy, entropy, and Gibb's free energy using the Gibb's free energy equation:

Keep in mind that temperature must be in Kelvin. Since we know enthalpy and Gibb's free energy for the reaction, we can solve for the entropy change:


We can relate enthalpy, entropy, and Gibb's free energy using the Gibb's free energy equation:
Keep in mind that temperature must be in Kelvin. Since we know enthalpy and Gibb's free energy for the reaction, we can solve for the entropy change:
Compare your answer with the correct one above
Which of the following options indicates that a chemical reaction is unfavorable?
Which of the following options indicates that a chemical reaction is unfavorable?
The sign of 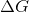 indicates the direction of a chemical reaction and determines if a reaction is spontaneous or not. A chemical reaction in which
indicates the direction of a chemical reaction and determines if a reaction is spontaneous or not. A chemical reaction in which  is considered to be a spontaneous reaction because it will proceed without requiring any outside energy. To approach this problem, we must consider the equation for the Gibbs free energy of a reaction,
is considered to be a spontaneous reaction because it will proceed without requiring any outside energy. To approach this problem, we must consider the equation for the Gibbs free energy of a reaction,  . A reaction is considered to be favorable when
. A reaction is considered to be favorable when 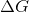 is negative. The second law of thermodynamics indicates that the entropy of the universe is spontaneously increasing. This corresponds to a positive
is negative. The second law of thermodynamics indicates that the entropy of the universe is spontaneously increasing. This corresponds to a positive  .
.
The sign of 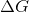



Compare your answer with the correct one above
Use the following values for water as needed.




If burning wood releases  of heat energy per gram of wood consumed, what mass of wood must be consumed to heat
of heat energy per gram of wood consumed, what mass of wood must be consumed to heat  of water from
of water from  to
to  , and then to convert it to water vapor?
, and then to convert it to water vapor?
Use the following values for water as needed.
If burning wood releases 



There are two processes requiring added heat in this problem:
1. Raising the temperature of the liquid water from  to
to  (use
(use  )
)
2. Boiling the water at a constant temperature of  (use
(use  )
)
To use either of these equation, we need to find the mass of the water using the relation between mass, density, and volume.

Use this mass with the given specific heat and temperatures to find the heat for part 1 of the process.

Then, use the mass with the given heat of vaporization to find the energy needed to convert the water to water vapor.

Sum the energies for step 1 and step 2.

This is the total amount of energy needed from the burning wood. Use stoichiometry to find the grams of wood needed to produce this amount of energy.

There are two processes requiring added heat in this problem:
1. Raising the temperature of the liquid water from 


2. Boiling the water at a constant temperature of 

To use either of these equation, we need to find the mass of the water using the relation between mass, density, and volume.
Use this mass with the given specific heat and temperatures to find the heat for part 1 of the process.
Then, use the mass with the given heat of vaporization to find the energy needed to convert the water to water vapor.
Sum the energies for step 1 and step 2.
This is the total amount of energy needed from the burning wood. Use stoichiometry to find the grams of wood needed to produce this amount of energy.
Compare your answer with the correct one above
Calculate the specific heat of  of metal that requires
of metal that requires  of heat energy to raise its temperature from
of heat energy to raise its temperature from  to
to  ?
?
Calculate the specific heat of 



Specific heat is the amount of heat needed to raise 1 gram of a substance by 1oC. Calorimeters used for these types of experiments because they are designed to be well-insulated, so no heat is gained from or lost to the surroundings.



Specific heat is the amount of heat needed to raise 1 gram of a substance by 1oC. Calorimeters used for these types of experiments because they are designed to be well-insulated, so no heat is gained from or lost to the surroundings.
Compare your answer with the correct one above