Oxidation-Reduction Reactions - GRE Subject Test: Chemistry
Card 0 of 2
Methane combusts in the presence of oxygen according to the following reaction:

Which of the following statements is true concerning the reaction?
Methane combusts in the presence of oxygen according to the following reaction:
Which of the following statements is true concerning the reaction?
By comparing the oxidation number of an atom as a reactant and its oxidation number as a product, we can determine if the atom has been oxidized or reduced. In increase in oxidation number indicates a loss of electrons, or oxidation. A decrease in oxidation number signals a gain of electrons, or reduction.
For electrochemistry, you should familiarize yourself with the traditional oxidation states of hydrogen  , halogens
, halogens  , oxygen
, oxygen  , and elemental atoms
, and elemental atoms  .
.
Carbon is initially in the form of methane, meaning that it is attached to four hydrogen atoms. The molecule is neutral, and each hydrogen has an oxidation number of  . Carbon must have an initial oxidation state of
. Carbon must have an initial oxidation state of  in order to balance the molecular charge.
in order to balance the molecular charge.
In the a product, carbon is attached to two oxygens, each with a charge of 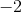 . Again, the molecule is neutral, so carbon must balance these charges. This means that carbon's final oxidation state is
. Again, the molecule is neutral, so carbon must balance these charges. This means that carbon's final oxidation state is  .
.
Since carbon went from an oxidation state of  to
to 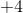 , we can conclude that carbon has been oxidized in the reaction.
, we can conclude that carbon has been oxidized in the reaction.
By comparing the oxidation number of an atom as a reactant and its oxidation number as a product, we can determine if the atom has been oxidized or reduced. In increase in oxidation number indicates a loss of electrons, or oxidation. A decrease in oxidation number signals a gain of electrons, or reduction.
For electrochemistry, you should familiarize yourself with the traditional oxidation states of hydrogen 



Carbon is initially in the form of methane, meaning that it is attached to four hydrogen atoms. The molecule is neutral, and each hydrogen has an oxidation number of 

In the a product, carbon is attached to two oxygens, each with a charge of 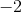

Since carbon went from an oxidation state of 
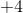
Compare your answer with the correct one above
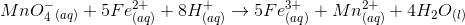
Based on the chemical equation given, which of the following compounds underwent reduction?
Based on the chemical equation given, which of the following compounds underwent reduction?
The type of chemical reaction given is called an oxidation-reduction reaction. In this type of reaction, there is a transfer of electrons from one element to another. The species gaining an electron is said to be reduced and the species losing an electron is oxidized. Manganese ion goes from a  to a
to a  oxidation state. Therefore, manganese is reduced in the chemical reaction because it gains electrons from the compound being oxidized (iron).
oxidation state. Therefore, manganese is reduced in the chemical reaction because it gains electrons from the compound being oxidized (iron).
The type of chemical reaction given is called an oxidation-reduction reaction. In this type of reaction, there is a transfer of electrons from one element to another. The species gaining an electron is said to be reduced and the species losing an electron is oxidized. Manganese ion goes from a 

Compare your answer with the correct one above