Gibbs Free Energy - GRE Subject Test: Chemistry
Card 0 of 2
At  , a reaction has a Gibb's free energy change of
, a reaction has a Gibb's free energy change of  . If the enthalpy change of the reaction is
. If the enthalpy change of the reaction is  , what is the entropy change of the reaction?
, what is the entropy change of the reaction?
At 


We can relate enthalpy, entropy, and Gibb's free energy using the Gibb's free energy equation:

Keep in mind that temperature must be in Kelvin. Since we know enthalpy and Gibb's free energy for the reaction, we can solve for the entropy change:


We can relate enthalpy, entropy, and Gibb's free energy using the Gibb's free energy equation:
Keep in mind that temperature must be in Kelvin. Since we know enthalpy and Gibb's free energy for the reaction, we can solve for the entropy change:
Compare your answer with the correct one above
Which of the following options indicates that a chemical reaction is unfavorable?
Which of the following options indicates that a chemical reaction is unfavorable?
The sign of 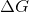 indicates the direction of a chemical reaction and determines if a reaction is spontaneous or not. A chemical reaction in which
indicates the direction of a chemical reaction and determines if a reaction is spontaneous or not. A chemical reaction in which  is considered to be a spontaneous reaction because it will proceed without requiring any outside energy. To approach this problem, we must consider the equation for the Gibbs free energy of a reaction,
is considered to be a spontaneous reaction because it will proceed without requiring any outside energy. To approach this problem, we must consider the equation for the Gibbs free energy of a reaction,  . A reaction is considered to be favorable when
. A reaction is considered to be favorable when 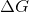 is negative. The second law of thermodynamics indicates that the entropy of the universe is spontaneously increasing. This corresponds to a positive
is negative. The second law of thermodynamics indicates that the entropy of the universe is spontaneously increasing. This corresponds to a positive  .
.
The sign of 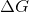



Compare your answer with the correct one above