Reaction Chemistry - GRE Subject Test: Chemistry
Card 0 of 20
What is the net ionic equation for the ion exchange reaction between ferrous sulfate and calcium iodide? Assume all compounds are soluble.
What is the net ionic equation for the ion exchange reaction between ferrous sulfate and calcium iodide? Assume all compounds are soluble.
First, we must know what ferrous sulfate is. Ferrous refers to  , and sulfate has the formula
, and sulfate has the formula  . When we combine the two together we get
. When we combine the two together we get  .
.
Calcium is a divatent cation and iodide is a monovalent anion, so their salt is  . The ion exchange reaction is then:
. The ion exchange reaction is then:

First, we must know what ferrous sulfate is. Ferrous refers to 


Calcium is a divatent cation and iodide is a monovalent anion, so their salt is 
Compare your answer with the correct one above
Select the net ionic equation from this molecular reaction:

Select the net ionic equation from this molecular reaction:
The net ionic equation is derived by removing all spectator ions from the total ionic equation (in which all ions are listed). To put it another way, the net ionic equation involves only the ions that participate in a reaction which, in this case, is the precipitation of barium sulfate.
Begin by writing all aqueous compounds in their dissociated (ionic) forms.


Cancel out any ions that appear in equal quantities on both sides of the equation. In this case, we can cancel the nitrate and potassium ions.

This is our net ionic equation.
The net ionic equation is derived by removing all spectator ions from the total ionic equation (in which all ions are listed). To put it another way, the net ionic equation involves only the ions that participate in a reaction which, in this case, is the precipitation of barium sulfate.
Begin by writing all aqueous compounds in their dissociated (ionic) forms.
Cancel out any ions that appear in equal quantities on both sides of the equation. In this case, we can cancel the nitrate and potassium ions.
This is our net ionic equation.
Compare your answer with the correct one above
What is the balanced chemical equation for the combustion of butane  ?
?
What is the balanced chemical equation for the combustion of butane 
Combustion is the chemical reaction of a hydrocarbon with molecular oxygen, and it always produces carbon dioxide and water. Knowing the reactants and products, the unbalanced equation must be:

We start by balancing the hydrogens. Since there are 10 on the left and only 2 on the right, we put a coefficient of 5 on water.

Similarly, we balance carbons by putting a 4 on the carbon dioxide.

To find the number of oxygens on the right, we multiply the 4 coefficient by the 2 subscript on O (which gets us 8 oxygens) and then add the 5 oxygens from the 5 water molecules to get a total of 13. The needed coefficient for  on the left would then have to be 13/2.
on the left would then have to be 13/2.

Because fractional coefficients are not allowed, we mutiply every coefficient by 2 to find our final reaction:


Combustion is the chemical reaction of a hydrocarbon with molecular oxygen, and it always produces carbon dioxide and water. Knowing the reactants and products, the unbalanced equation must be:
We start by balancing the hydrogens. Since there are 10 on the left and only 2 on the right, we put a coefficient of 5 on water.
Similarly, we balance carbons by putting a 4 on the carbon dioxide.
To find the number of oxygens on the right, we multiply the 4 coefficient by the 2 subscript on O (which gets us 8 oxygens) and then add the 5 oxygens from the 5 water molecules to get a total of 13. The needed coefficient for 
Because fractional coefficients are not allowed, we mutiply every coefficient by 2 to find our final reaction:
Compare your answer with the correct one above
Determine whether or not solid aluminum reacts with aqueous zinc chloride. If it does, determine the balanced equation for the reaction.
Determine whether or not solid aluminum reacts with aqueous zinc chloride. If it does, determine the balanced equation for the reaction.
When we check the activity series, it is fairly easy to see that aluminum metal is more reactive than zinc metal. So, in this case, the two metals undergo a redox reaction, where the aqueous  is reduced to solid
is reduced to solid  , and the solid
, and the solid  is oxidized to aqueous
is oxidized to aqueous  . These charges are the common oxidation states for zinc and aluminum and should be memorized.
. These charges are the common oxidation states for zinc and aluminum and should be memorized.
Because  is the new species, it bonds with 3
is the new species, it bonds with 3  ions. The unbalanced equation is:
ions. The unbalanced equation is:

We note that there are 2 chlorine atoms on the left and 3 chlorine atoms on the right. To balance, we use a 3 coefficient on the left and a 2 coefficient on the right. This gives a total of 6 chlorine atoms on eahc side.

However, now we have also increased the amounts of zinc and aluminum. We copy the necessary coefficients to balance those—2 for aluminum on the left, 3 for zinc on the right—and we are done:

When we check the activity series, it is fairly easy to see that aluminum metal is more reactive than zinc metal. So, in this case, the two metals undergo a redox reaction, where the aqueous 



Because 

We note that there are 2 chlorine atoms on the left and 3 chlorine atoms on the right. To balance, we use a 3 coefficient on the left and a 2 coefficient on the right. This gives a total of 6 chlorine atoms on eahc side.
However, now we have also increased the amounts of zinc and aluminum. We copy the necessary coefficients to balance those—2 for aluminum on the left, 3 for zinc on the right—and we are done:
Compare your answer with the correct one above
What will be the coefficient for  once the following equation is balanced?
once the following equation is balanced?

What will be the coefficient for 
In the unbalanced equation from the question, the reactant side contains two hydrogen atoms and one oxygen atom. On the product side of the equation, there are four hydrogens and two oxygen atoms. So for every oxygen atom there are two hydrogen atoms giving a ratio of  (oxygen : hydrogen). Putting a coefficient of two in front of the water molecule on the reactant side of the equation balances the equation. Therefore, there are four hydrogen and two oxygen atoms on both sides of the balanced chemical equation.
(oxygen : hydrogen). Putting a coefficient of two in front of the water molecule on the reactant side of the equation balances the equation. Therefore, there are four hydrogen and two oxygen atoms on both sides of the balanced chemical equation.
In the unbalanced equation from the question, the reactant side contains two hydrogen atoms and one oxygen atom. On the product side of the equation, there are four hydrogens and two oxygen atoms. So for every oxygen atom there are two hydrogen atoms giving a ratio of 
Compare your answer with the correct one above

When 15.5 grams of  is used in the given reaction, how many moles of
is used in the given reaction, how many moles of  is produced?
is produced?
When 15.5 grams of 

We must first calculate the molecular weight of  to use as a conversion factor:
to use as a conversion factor:


For every mole of  reacted, one mole of
reacted, one mole of  is produced.
is produced.
Therefore, 0.1092 moles of  is produced.
is produced.
We must first calculate the molecular weight of 
For every mole of 

Therefore, 0.1092 moles of 
Compare your answer with the correct one above

When 20.0 grams of  is used in the given reaction, how many moles of
is used in the given reaction, how many moles of  is produced?
is produced?
When 20.0 grams of 

We must first calculate the molecular weight of  to use as a conversion factor:
to use as a conversion factor:


For every 1 mole of  reacted, 2 moles of
reacted, 2 moles of  is produced.
is produced.
Therefore,the number of moles of  produced will be double the number of moles of
produced will be double the number of moles of  used up in the reaction:
used up in the reaction:

We must first calculate the molecular weight of 
For every 1 mole of 

Therefore,the number of moles of 

Compare your answer with the correct one above

What is the smallest whole-number coefficient that can be designated to the water molecule that would balance the chemical equation given?
What is the smallest whole-number coefficient that can be designated to the water molecule that would balance the chemical equation given?
The number of atoms on the reactant side of the chemical equation are:

The number of atoms on the product side of the chemical equation are:

In a balanced chemical equation, the total number of atoms of each element must be equal on the reactant and product side of the chemical equation. There is one more oxygen and two more hydrogen atoms on the reactant side of the chemical equation. Giving the water molecule on the product side of the chemical equation would balance the chemical equation so that both sides contain six oxygens and five hydrogens:
The number of atoms on the reactant side of the chemical equation are:
The number of atoms on the product side of the chemical equation are:
In a balanced chemical equation, the total number of atoms of each element must be equal on the reactant and product side of the chemical equation. There is one more oxygen and two more hydrogen atoms on the reactant side of the chemical equation. Giving the water molecule on the product side of the chemical equation would balance the chemical equation so that both sides contain six oxygens and five hydrogens:
Compare your answer with the correct one above

What is the smallest whole number coefficient that can be designated to the  molecule that would balance the chemical equation given?
molecule that would balance the chemical equation given?
What is the smallest whole number coefficient that can be designated to the 
The process of balancing a chemical equation is called "balancing by inspection" which is done by trial and error. The easiest way to approach the problem given is by balancing the species containing an element with the most complicated formula occurring in one reactant and one product. In this case, we can either begin by balancing the carbon or hydrogen in the propane molecule. Let's start with the carbon by adding a coefficient of 3 to the carbon dioxide molecule on the product side of the equation which gives:

Let's now balance the hydrogens by adding a coefficient of 4 to the product side of the equation:

Lastly, we can balance the number of oxygens in the reaction:

The process of balancing a chemical equation is called "balancing by inspection" which is done by trial and error. The easiest way to approach the problem given is by balancing the species containing an element with the most complicated formula occurring in one reactant and one product. In this case, we can either begin by balancing the carbon or hydrogen in the propane molecule. Let's start with the carbon by adding a coefficient of 3 to the carbon dioxide molecule on the product side of the equation which gives:
Let's now balance the hydrogens by adding a coefficient of 4 to the product side of the equation:
Lastly, we can balance the number of oxygens in the reaction:
Compare your answer with the correct one above

If  of
of  is used for the reaction given, how many grams of
is used for the reaction given, how many grams of  was formed?
was formed?
If 


Based on the molecular equation, for every 1 mole of  reacted, 1 mole of
reacted, 1 mole of  is formed as a product. Using dimensional analysis, we can use this relationship to determine the moles of
is formed as a product. Using dimensional analysis, we can use this relationship to determine the moles of  reacted:
reacted:

We must calculate the molecular weight of  :
:

To calculate the number of grams of  , we need to convert the number of moles of
, we need to convert the number of moles of  to grams using the molecular weight as a conversion factor.
to grams using the molecular weight as a conversion factor.

Based on the molecular equation, for every 1 mole of 


We must calculate the molecular weight of 
To calculate the number of grams of 

Compare your answer with the correct one above

If  of
of  is used for the reaction given, how many grams of
is used for the reaction given, how many grams of  was formed?
was formed?
If 


Based on the molecular equation, for every 4 moles of  reacted, 2 moles of
reacted, 2 moles of  is formed as a product:
is formed as a product:

We must calculate the molecular weight of  :
:

To calculate the number of grams of  , we need to convert the number of moles of
, we need to convert the number of moles of  to grams using the molecular weight as a conversion factor.
to grams using the molecular weight as a conversion factor.

Based on the molecular equation, for every 4 moles of 

We must calculate the molecular weight of 
To calculate the number of grams of 

Compare your answer with the correct one above

If  of
of  is formed during the reaction given, how many grams of
is formed during the reaction given, how many grams of  was used?
was used?
If 


Based on the molecular equation, for every 2 moles of  formed, 1 mole of
formed, 1 mole of  is reacted:
is reacted:

We must calculate the molecular weight of  :
:

To calculate the number of grams of  , we need to convert the number of moles of
, we need to convert the number of moles of  to grams using the molecular weight as a conversion factor.
to grams using the molecular weight as a conversion factor.

Based on the molecular equation, for every 2 moles of 

We must calculate the molecular weight of 
To calculate the number of grams of 

Compare your answer with the correct one above

If  of
of  is formed during the reaction given, how many grams of
is formed during the reaction given, how many grams of  was used?
was used?
If 


Based on the molecular equation, for every 3 moles of  formed, 1 mole of
formed, 1 mole of  is reacted:
is reacted:

We must calculate the molecular weight of  :
:

To calculate the number of grams of  , we need to convert the number of moles of
, we need to convert the number of moles of  to grams using the molecular weight as a conversion factor.
to grams using the molecular weight as a conversion factor.

Based on the molecular equation, for every 3 moles of 

We must calculate the molecular weight of 
To calculate the number of grams of 

Compare your answer with the correct one above
Which of the following is true regarding the solubility product constant,  , for a reaction in the form:
, for a reaction in the form:

Which of the following is true regarding the solubility product constant, 
To determine the solubility product constant, we only need the concentrations and coefficients of the ions. The effective concentration of any pure substance (solid, liquid, or gas) is equal to one by definition, so does not influence the value of  . The equation for the solubility product constant of this reaction is:
. The equation for the solubility product constant of this reaction is:
![K_{sp}=[C]^c[D]^d](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/100575/gif.latex)
The units of the solubility product constant will depend on the coefficients of the products. 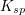 will be a constant for the reaction, and will not change as more solid dissolves or as the reaction progresses.
will be a constant for the reaction, and will not change as more solid dissolves or as the reaction progresses.
To determine the solubility product constant, we only need the concentrations and coefficients of the ions. The effective concentration of any pure substance (solid, liquid, or gas) is equal to one by definition, so does not influence the value of 
The units of the solubility product constant will depend on the coefficients of the products. 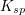
Compare your answer with the correct one above
Methane combusts in the presence of oxygen according to the following reaction:

Which of the following statements is true concerning the reaction?
Methane combusts in the presence of oxygen according to the following reaction:
Which of the following statements is true concerning the reaction?
By comparing the oxidation number of an atom as a reactant and its oxidation number as a product, we can determine if the atom has been oxidized or reduced. In increase in oxidation number indicates a loss of electrons, or oxidation. A decrease in oxidation number signals a gain of electrons, or reduction.
For electrochemistry, you should familiarize yourself with the traditional oxidation states of hydrogen  , halogens
, halogens  , oxygen
, oxygen  , and elemental atoms
, and elemental atoms  .
.
Carbon is initially in the form of methane, meaning that it is attached to four hydrogen atoms. The molecule is neutral, and each hydrogen has an oxidation number of  . Carbon must have an initial oxidation state of
. Carbon must have an initial oxidation state of  in order to balance the molecular charge.
in order to balance the molecular charge.
In the a product, carbon is attached to two oxygens, each with a charge of 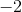 . Again, the molecule is neutral, so carbon must balance these charges. This means that carbon's final oxidation state is
. Again, the molecule is neutral, so carbon must balance these charges. This means that carbon's final oxidation state is  .
.
Since carbon went from an oxidation state of  to
to 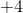 , we can conclude that carbon has been oxidized in the reaction.
, we can conclude that carbon has been oxidized in the reaction.
By comparing the oxidation number of an atom as a reactant and its oxidation number as a product, we can determine if the atom has been oxidized or reduced. In increase in oxidation number indicates a loss of electrons, or oxidation. A decrease in oxidation number signals a gain of electrons, or reduction.
For electrochemistry, you should familiarize yourself with the traditional oxidation states of hydrogen 



Carbon is initially in the form of methane, meaning that it is attached to four hydrogen atoms. The molecule is neutral, and each hydrogen has an oxidation number of 

In the a product, carbon is attached to two oxygens, each with a charge of 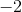

Since carbon went from an oxidation state of 
Compare your answer with the correct one above
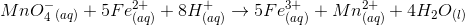
Based on the chemical equation given, which of the following compounds underwent reduction?
Based on the chemical equation given, which of the following compounds underwent reduction?
The type of chemical reaction given is called an oxidation-reduction reaction. In this type of reaction, there is a transfer of electrons from one element to another. The species gaining an electron is said to be reduced and the species losing an electron is oxidized. Manganese ion goes from a  to a
to a  oxidation state. Therefore, manganese is reduced in the chemical reaction because it gains electrons from the compound being oxidized (iron).
oxidation state. Therefore, manganese is reduced in the chemical reaction because it gains electrons from the compound being oxidized (iron).
The type of chemical reaction given is called an oxidation-reduction reaction. In this type of reaction, there is a transfer of electrons from one element to another. The species gaining an electron is said to be reduced and the species losing an electron is oxidized. Manganese ion goes from a 

Compare your answer with the correct one above
Consider the reaction reaction below.

A student allows the system to reach equilibrium and then removes two moles of hydrogen gas. Which of the following will be a result?
Consider the reaction reaction below.
A student allows the system to reach equilibrium and then removes two moles of hydrogen gas. Which of the following will be a result?
According to Le Chatelier's principle, when a system at equilibrium is disturbed, the system will react to restore equilibrium. In other words, it will seek to undo the stress. Here, if hydrogen gas is removed, the reaction will shift toward the reactants to re-form it. In the process, more nitrogen will be produced.
According to Le Chatelier's principle, when a system at equilibrium is disturbed, the system will react to restore equilibrium. In other words, it will seek to undo the stress. Here, if hydrogen gas is removed, the reaction will shift toward the reactants to re-form it. In the process, more nitrogen will be produced.
Compare your answer with the correct one above


The initial concentrations of this reaction are listed below.
![[CH_{3}OH] = 2.5M, [CO] = 1.3M, [H_{2}] =0.6M.](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/115362/gif.latex)
Based on these initial concentrations, which statement is true?
The initial concentrations of this reaction are listed below.
Based on these initial concentrations, which statement is true?
When given initial concentrations, we can determine the reaction quotient (Q) of the reaction. The reaction quotient is given by the same equation as the equilibrium constant (concentration of products divided by concentration of reactants), but its value will fluctuate as the system reacts, whereas the equilibrium constant is based on equilibrium concentrations.
By comparing the reaction quotient to the equilibrium constant, we can determine in which direction the reaction will proceed initially.
![Q\ \text{or}\ K_{eq}=\frac{[CH_3OH]}{[H_2]^2[CO]}](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/183372/gif.latex)
The reaction quotient with the beginning concentrations is written below.
![\frac{[2.5]}{[0.6]^{2}[1.3]} = 5.3](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/86003/gif.latex)
This shows that the ratio of products to reactants is less than the equilibrium constant. Since Q is less than Keq in the beginning, we conclude that the reaction will proceed forward until Q is equal to Keq. In this manner, the denominator (reactants) will decrease and the numerator (products) will increase, causing Q to become closer to Keq.
When given initial concentrations, we can determine the reaction quotient (Q) of the reaction. The reaction quotient is given by the same equation as the equilibrium constant (concentration of products divided by concentration of reactants), but its value will fluctuate as the system reacts, whereas the equilibrium constant is based on equilibrium concentrations.
By comparing the reaction quotient to the equilibrium constant, we can determine in which direction the reaction will proceed initially.
The reaction quotient with the beginning concentrations is written below.
This shows that the ratio of products to reactants is less than the equilibrium constant. Since Q is less than Keq in the beginning, we conclude that the reaction will proceed forward until Q is equal to Keq. In this manner, the denominator (reactants) will decrease and the numerator (products) will increase, causing Q to become closer to Keq.
Compare your answer with the correct one above
A saturated solution of scandium hydroxide, which also contains solid scandium hydroxide, is treated with 0.1N HCl. The addition of acid will __________ the solubility of scandium hydroxide because of __________.
A saturated solution of scandium hydroxide, which also contains solid scandium hydroxide, is treated with 0.1N HCl. The addition of acid will __________ the solubility of scandium hydroxide because of __________.
Scandium hydroxide dissociates according to this reaction.

As 0.1N HCl is added, it will quantitatively react with hydroxide anions to produce ScCl3 and water.

Hydroxide will be removed from the above equilibrium, and the system will compensate by shifting the equilibrium to the right, according Le Chatelier's principle. As the equilibrium shifts to the right, more solid scandium hydroxide is hydrolyzed, resulting in increased solubility.
Scandium hydroxide dissociates according to this reaction.
As 0.1N HCl is added, it will quantitatively react with hydroxide anions to produce ScCl3 and water.
Hydroxide will be removed from the above equilibrium, and the system will compensate by shifting the equilibrium to the right, according Le Chatelier's principle. As the equilibrium shifts to the right, more solid scandium hydroxide is hydrolyzed, resulting in increased solubility.
Compare your answer with the correct one above
A system contains iodine atoms that are in equilibrium with respect to the reaction below:

The volume of the system is suddenly reduced, leading to an increase in pressure. What effect will this have on the reaction?
A system contains iodine atoms that are in equilibrium with respect to the reaction below:
The volume of the system is suddenly reduced, leading to an increase in pressure. What effect will this have on the reaction?
Since the system is originally at equilibrium and a stress is applied, Le Chatelier's principle is to be considered. By increasing the total pressure, the reaction will move in the direction toward which there are less molecules of gas.

Looking at the original reaction there are two moles of gas on the left and only one on the right, indicating that the reaction will shift to the right if the pressure is increased.
Since the system is originally at equilibrium and a stress is applied, Le Chatelier's principle is to be considered. By increasing the total pressure, the reaction will move in the direction toward which there are less molecules of gas.
Looking at the original reaction there are two moles of gas on the left and only one on the right, indicating that the reaction will shift to the right if the pressure is increased.
Compare your answer with the correct one above