Nuclear Chemistry - GRE Subject Test: Chemistry
Card 0 of 20
What is the electron configuration of potassium after it obtains a +1 charge?
What is the electron configuration of potassium after it obtains a +1 charge?
Potassium (K) is orignially in the electron configuration of \[Ar\]4s1. To obtain a +1 charge it loses an electron, resulting in a configuration of \[Ar\].
Potassium (K) is orignially in the electron configuration of \[Ar\]4s1. To obtain a +1 charge it loses an electron, resulting in a configuration of \[Ar\].
Compare your answer with the correct one above
What is the electron configuration of Fe+?
What is the electron configuration of Fe+?
When an element loses an electron it is generally taken away from the highest electron shell. The electron configuration of iron (Fe) is ![[Ar]4s^23d^6](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/25907/gif.latex) . The 4s orbital is farther away from the nucleus than the 3d orbital, therefore the electron configuration of Fe+ will be
. The 4s orbital is farther away from the nucleus than the 3d orbital, therefore the electron configuration of Fe+ will be ![[Ar]4s^13d^6](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/25908/gif.latex) .
.
When an element loses an electron it is generally taken away from the highest electron shell. The electron configuration of iron (Fe) is ![[Ar]4s^23d^6](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/25907/gif.latex)
![[Ar]4s^13d^6](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/25908/gif.latex)
Compare your answer with the correct one above
An atom with the electron configuration 1s22s22p6 could be any of the following except _________.
An atom with the electron configuration 1s22s22p6 could be any of the following except _________.
This particular configuration denotes a particle with ten total electrons. The sodium atom, with eleven electrons, is the only one listed that could not have this configuration. Ionized sodium, however, symbolized as Na+, does apply. (Be careful to distinguish neutral atoms and ions).
This particular configuration denotes a particle with ten total electrons. The sodium atom, with eleven electrons, is the only one listed that could not have this configuration. Ionized sodium, however, symbolized as Na+, does apply. (Be careful to distinguish neutral atoms and ions).
Compare your answer with the correct one above
Which of the following is the correct electronic configuration for vanadium?
Which of the following is the correct electronic configuration for vanadium?
When determining electronic configuration, the answer is made much easier by starting with the next smallest noble gas in brackets. As a result, \[Ar\] is an appropriate way to incorporate every previous electron before argon.
After argon, vanadium has five other electrons to distribute, and because vanadium is a transitional element, it will fill its 3d subshells before filling the 4p subshells. The 4s subshell is filled first, and the last three electrons are placed into the 3d subshells.
\[Ar\]3d34s2
When determining electronic configuration, the answer is made much easier by starting with the next smallest noble gas in brackets. As a result, \[Ar\] is an appropriate way to incorporate every previous electron before argon.
After argon, vanadium has five other electrons to distribute, and because vanadium is a transitional element, it will fill its 3d subshells before filling the 4p subshells. The 4s subshell is filled first, and the last three electrons are placed into the 3d subshells.
\[Ar\]3d34s2
Compare your answer with the correct one above
What is the correct electronic structure for  ?
?
What is the correct electronic structure for 
Were this question asking for the electronic structure of magnesium (Mg) in its ground state,  would be the correct answer; however, the
would be the correct answer; however, the  charge on
charge on  means that the molecule shed two valence electrons to achieve a more stable orbital. Those electrons will be shed from the outermost valence shell, which in this case is the
means that the molecule shed two valence electrons to achieve a more stable orbital. Those electrons will be shed from the outermost valence shell, which in this case is the  shell; therefore,
shell; therefore,  is correct.
is correct.
Note that the ground state of magnesium will have twelve electrons (the same as its atomic number), while the ion will have ten.
Were this question asking for the electronic structure of magnesium (Mg) in its ground state, 




Note that the ground state of magnesium will have twelve electrons (the same as its atomic number), while the ion will have ten.
Compare your answer with the correct one above
An electron in which of the following orbitals is closest to the nucleus?
An electron in which of the following orbitals is closest to the nucleus?
Nuclear orbitals will always fill from the innermost to the outermost subshells. Using the  rule, we can approximate the order in which these orbitals will fill. Because electrons fill starting with the centermost orbitals, the electron that is closest to the nucleus will belong to the orbital that fills first.
rule, we can approximate the order in which these orbitals will fill. Because electrons fill starting with the centermost orbitals, the electron that is closest to the nucleus will belong to the orbital that fills first.
 corresponds to the principle quantum number, the first number in the given orbital location.
corresponds to the principle quantum number, the first number in the given orbital location.  is the azimuthal quantum number, and dictates the shape of the orbital.
is the azimuthal quantum number, and dictates the shape of the orbital.




5s and 3d produce the same number from the  equation, but in the event of a tie we always pick the orbital with the lowest letter (s < p < d < f). The innermost orbital will be 5s.
equation, but in the event of a tie we always pick the orbital with the lowest letter (s < p < d < f). The innermost orbital will be 5s.




Nuclear orbitals will always fill from the innermost to the outermost subshells. Using the 





5s and 3d produce the same number from the 
Compare your answer with the correct one above
Which of the following species is represented by the given electron configuration?

Which of the following species is represented by the given electron configuration?
Due to the phenomenon of half-orbital stability in the transition metals, electrons can easily move between 4s and 3d orbitals. The atom achieves greater stability from having only one atom in the 4s orbital, allowing a half-filled 3d orbital, as opposed to a full 4s orbital and four electrons in the 3d subshell.
For elements like chromium and copper, which could have valence shell configurations of 4s23d4 and _4s_23d9, respectively, an electron from the 4s orbital jumps down to the 3d orbital to harness added stability from the half-filled orbital. The given electron configuration is that of chromium.
Note that you can also solve this question by counting the electrons to determine the atomic number. In this case, the electrons add up to 24, indicating the twenty-fourth element: chromium.
Due to the phenomenon of half-orbital stability in the transition metals, electrons can easily move between 4s and 3d orbitals. The atom achieves greater stability from having only one atom in the 4s orbital, allowing a half-filled 3d orbital, as opposed to a full 4s orbital and four electrons in the 3d subshell.
For elements like chromium and copper, which could have valence shell configurations of 4s23d4 and _4s_23d9, respectively, an electron from the 4s orbital jumps down to the 3d orbital to harness added stability from the half-filled orbital. The given electron configuration is that of chromium.
Note that you can also solve this question by counting the electrons to determine the atomic number. In this case, the electrons add up to 24, indicating the twenty-fourth element: chromium.
Compare your answer with the correct one above
Which element has the most valence electrons?
Which element has the most valence electrons?
Valence electrons will be housed in the outer shell (highest numbered orbital) of the electron configuration.
Calcium: ![[Ar]4s^2](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/96885/gif.latex)
Magnesium: ![[Ne]3s^ 2](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/123245/gif.latex)
Zinc: ![[Ar]4s^23d^{10}](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/96886/gif.latex)
Copper: ![[Ar]4s^13d^{10}](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/123246/gif.latex)
Arsenic: ![[Ar]4s^23d^{10}4p^3](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/96887/gif.latex)
Calcium, magnesium, and zinc all have two valence electrons in their highest energy orbital. Copper has only one valence electron. Arsenic has five total valence electrons in the fourth shell.
Valence electrons will be housed in the outer shell (highest numbered orbital) of the electron configuration.
Calcium:
Magnesium:
Zinc:
Copper:
Arsenic:
Calcium, magnesium, and zinc all have two valence electrons in their highest energy orbital. Copper has only one valence electron. Arsenic has five total valence electrons in the fourth shell.
Compare your answer with the correct one above
What is the correct electron configuration of  ?
?
What is the correct electron configuration of 
An atom that has a charge of  has lost two electrons. The two lost are always the valence electrons, which are most easily taken from the molecule. Strontium has a normal electron configuration with 38 electrons. In shorthand notation, this would be:
has lost two electrons. The two lost are always the valence electrons, which are most easily taken from the molecule. Strontium has a normal electron configuration with 38 electrons. In shorthand notation, this would be:
![[Kr]5s^2](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/96914/gif.latex)
Losing two valence electrons will remove the 5s electrons. This leave the configuration as:
![[Kr]](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/132321/gif.latex)
This makes sense because krypton has a stable valence octet. Strontium is stable as an ion, meaning that it will also have an octet, allowing it to match the configuration for krypton.
An atom that has a charge of 
Losing two valence electrons will remove the 5s electrons. This leave the configuration as:
This makes sense because krypton has a stable valence octet. Strontium is stable as an ion, meaning that it will also have an octet, allowing it to match the configuration for krypton.
Compare your answer with the correct one above
Which two elements have the same number of electrons in the 3d shell?
Which two elements have the same number of electrons in the 3d shell?
Valence electrons in the d subshell can be odd because a half-filled d orbital is more stable than one with three or four electrons. While strontium, titanium, and vanadium have two electrons in their 4s orbitals, chromium has one in the 4s shell and puts five in the 3d shell.
![Cr:\ [Ar]4s^13d^5](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/96915/gif.latex)
This creates a half-filled 4s and 3d shells, which are more stable than a full 4s shell and partial 3d shell. Chromium and manganese thus have the same number of 3d electrons: five.
![Mn: [Ar]4s^23d^5](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/110626/gif.latex)
Valence electrons in the d subshell can be odd because a half-filled d orbital is more stable than one with three or four electrons. While strontium, titanium, and vanadium have two electrons in their 4s orbitals, chromium has one in the 4s shell and puts five in the 3d shell.
This creates a half-filled 4s and 3d shells, which are more stable than a full 4s shell and partial 3d shell. Chromium and manganese thus have the same number of 3d electrons: five.
Compare your answer with the correct one above
Which of the following is the correct notation for a sodium ion?
Which of the following is the correct notation for a sodium ion?
A neutral atom of sodium would contain eleven electrons to balance out the charge of the eleven protons in the nucleus. We are asked, however, for the configuration of a sodium ion. Sodium is an alkali metal, meaning that it will ionize by losing only one electron, gaining a charge of  . By losing one electron, sodium drops from having eleven electrons to ten. We will need to select the answer that shows an electron removed form the outermost shell.
. By losing one electron, sodium drops from having eleven electrons to ten. We will need to select the answer that shows an electron removed form the outermost shell.
Neutral sodium: 
Sodium ion: 
A neutral atom of sodium would contain eleven electrons to balance out the charge of the eleven protons in the nucleus. We are asked, however, for the configuration of a sodium ion. Sodium is an alkali metal, meaning that it will ionize by losing only one electron, gaining a charge of 
Neutral sodium:
Sodium ion:
Compare your answer with the correct one above
Which of the following is true about gaining and losing electrons in cobalt?
Which of the following is true about gaining and losing electrons in cobalt?
Cobalt is a transition metal; therefore, it is found in the D block of the periodic table. A ground state cobalt atom has an electron configuration of ![[Ar]4s^23d^7](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/157535/gif.latex) . The last orbitals that gain or lose electrons must be either the
. The last orbitals that gain or lose electrons must be either the  or
or  orbitals, since these are the orbitals with highest energy and located farthest from the nucleus.
orbitals, since these are the orbitals with highest energy and located farthest from the nucleus.
Recall that electrons are filled from orbitals of low energy to high energy. A  orbital has a lower energy than a
orbital has a lower energy than a  orbital. This means that when you are filling electrons, the last orbital you fill is the
orbital. This means that when you are filling electrons, the last orbital you fill is the  orbital. When you are assigning electrons to each orbital you assign two electrons to the
orbital. When you are assigning electrons to each orbital you assign two electrons to the  orbital and then the remaining seven electrons to the five
orbital and then the remaining seven electrons to the five  orbitals; therefore,
orbitals; therefore,  orbitals are filled last when gaining electrons.
orbitals are filled last when gaining electrons.
When an element loses electrons, the first orbital that loses electrons is the outermost orbital. This occurs because the attractive force of the nucleus on the electron will be weakest in the outermost orbital (because it is farthest away from nucleus); therefore, it will be easy to pull the electron away from the nucleus. In cobalt, the outermost orbital is the  orbital (because it has the highest shell number). This means that electrons will be lost from the
orbital (because it has the highest shell number). This means that electrons will be lost from the  orbital before the
orbital before the  orbital.
orbital.
Notice that the orbital that gains electron last is not the same orbital that loses electrons first. Gaining electrons is dependent on the energy of the orbital, and losing electrons is dependent on the location of the orbital. The highest energy orbital will gain electron last and the outermost orbital will lose electron first.
Cobalt is a transition metal; therefore, it is found in the D block of the periodic table. A ground state cobalt atom has an electron configuration of ![[Ar]4s^23d^7](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/157535/gif.latex)


Recall that electrons are filled from orbitals of low energy to high energy. A 





When an element loses electrons, the first orbital that loses electrons is the outermost orbital. This occurs because the attractive force of the nucleus on the electron will be weakest in the outermost orbital (because it is farthest away from nucleus); therefore, it will be easy to pull the electron away from the nucleus. In cobalt, the outermost orbital is the 


Notice that the orbital that gains electron last is not the same orbital that loses electrons first. Gaining electrons is dependent on the energy of the orbital, and losing electrons is dependent on the location of the orbital. The highest energy orbital will gain electron last and the outermost orbital will lose electron first.
Compare your answer with the correct one above
A certain transition metal has an electron configuration such that its  orbital only has one electron. What would be a valid conclusion about this element?
orbital only has one electron. What would be a valid conclusion about this element?
A certain transition metal has an electron configuration such that its 
The question states that the element is a transition element and it only has one electron in its  orbital. Recall that transition metals usually have two electrons in its
orbital. Recall that transition metals usually have two electrons in its  orbital; however, some transition metals lose one of the electrons from their
orbital; however, some transition metals lose one of the electrons from their  orbital and move it to one of their
orbital and move it to one of their  orbital. This occurs because of a phenomenon called half-shell stability. Half-shell stability states that an element is more stable when all the orbitals are half filled.
orbital. This occurs because of a phenomenon called half-shell stability. Half-shell stability states that an element is more stable when all the orbitals are half filled.
This is best exemplified by the transition metal chromium. The electron configuration of chromium, conventionally, would be ![[Ar]4s^23d^4](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/157634/gif.latex) . This means that chromium has two electrons in the
. This means that chromium has two electrons in the  orbital, one electron in four
orbital, one electron in four  orbitals, and an empty
orbitals, and an empty  orbital. Half-shell stability states that chromium is more stable if all five
orbital. Half-shell stability states that chromium is more stable if all five  orbitals have an electron, rather than having an empty
orbitals have an electron, rather than having an empty  orbital; therefore, an electron from the
orbital; therefore, an electron from the  orbital will be moved to the empty
orbital will be moved to the empty  orbital to fulfill half-shell stability. This gives one electron in the
orbital to fulfill half-shell stability. This gives one electron in the  orbital and one electron in each of the five
orbital and one electron in each of the five  orbitals.
orbitals.
Notice that half-shell stability is also observed in molybdenum ( ) and tungsten (
) and tungsten ( ). Molybdenum will have one electron in its
). Molybdenum will have one electron in its  orbital and one electron in each of its
orbital and one electron in each of its  orbitals, and tungsten will have one electron in its
orbitals, and tungsten will have one electron in its  orbital and one electron in each of its
orbital and one electron in each of its  orbitals.
orbitals.
The question states that the element is a transition element and it only has one electron in its 



This is best exemplified by the transition metal chromium. The electron configuration of chromium, conventionally, would be ![[Ar]4s^23d^4](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/157634/gif.latex)









Notice that half-shell stability is also observed in molybdenum (





Compare your answer with the correct one above
An oxygen atom and a fluorine atom have the same electron configuration as neon. Which of the following is true about these two atoms?
An oxygen atom and a fluorine atom have the same electron configuration as neon. Which of the following is true about these two atoms?
The question states that the oxygen and fluorine atoms have the same electron configuration as neon. The electron configuration for neon is  , with ten total electrons and eight valence electrons. To match this configuration, both the oxygen and the fluorine atom must have eight valence electrons (two in the
, with ten total electrons and eight valence electrons. To match this configuration, both the oxygen and the fluorine atom must have eight valence electrons (two in the  orbital and six in the
orbital and six in the  orbital).
orbital).
Recall that atoms in the oxygen group have six valence electrons, whereas halogens have seven valence electrons; therefore, oxygen has six and fluorine has seven valence electrons. To reach a total of eight valence electrons (octet) the oxygen atom must have gained two electrons, giving it a charge of  . Similarly, fluorine must have gained one electron, giving it a charge of
. Similarly, fluorine must have gained one electron, giving it a charge of  . Fluorine has half the of charge of oxygen because it gained half the amount of electrons to complete its octet.
. Fluorine has half the of charge of oxygen because it gained half the amount of electrons to complete its octet.
The question states that the oxygen and fluorine atoms have the same electron configuration as neon. The electron configuration for neon is 


Recall that atoms in the oxygen group have six valence electrons, whereas halogens have seven valence electrons; therefore, oxygen has six and fluorine has seven valence electrons. To reach a total of eight valence electrons (octet) the oxygen atom must have gained two electrons, giving it a charge of 

Compare your answer with the correct one above
How many electrons can fit in the  electron shell?
electron shell?
How many electrons can fit in the 
An 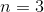 shell contains 3s, 3d, and 3p subshells. Any s shell can hold up to two electrons, any p shell can hold six electrons, and any d shell can hold ten electrons.
shell contains 3s, 3d, and 3p subshells. Any s shell can hold up to two electrons, any p shell can hold six electrons, and any d shell can hold ten electrons.
This question is best solved by using quantum numbers. We are given the principle quantum number. Using this, we can work down to determine how many electrons can fit in this shell. The rules for quantum numbers are given below.

If the principle quantum number is three, then the next quantum number can be zero, one, or two. These correspond to the s, p, and d subshells, respectively.
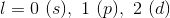
After that, the next quantum number describes the orbitals within the subshells. Each orbital can carry two electrons, according to the final quantum number.



In total, there are eighteen electrons allowed in all subshells of the third energy level.

An 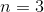
This question is best solved by using quantum numbers. We are given the principle quantum number. Using this, we can work down to determine how many electrons can fit in this shell. The rules for quantum numbers are given below.
If the principle quantum number is three, then the next quantum number can be zero, one, or two. These correspond to the s, p, and d subshells, respectively.
After that, the next quantum number describes the orbitals within the subshells. Each orbital can carry two electrons, according to the final quantum number.
In total, there are eighteen electrons allowed in all subshells of the third energy level.
Compare your answer with the correct one above
Consider the following isotope of thorium:

What is the identity of the product following three alpha decay reactions?
Consider the following isotope of thorium:
What is the identity of the product following three alpha decay reactions?
During alpha decay, an element emits a helium nucleus with 2 neutrons and 2 protons. Thus, the atomic mass of the new element is decreased by four, and the atomic number is decreased by two.
Three subsequent alpha decays result in a new element with an atomic mass of 232 - 3(4) = 220, and a new atomic number of 90 - 3(2) = 84.
Using the periodic table, we find the element with this atomic number is polonium (Po).
During alpha decay, an element emits a helium nucleus with 2 neutrons and 2 protons. Thus, the atomic mass of the new element is decreased by four, and the atomic number is decreased by two.
Three subsequent alpha decays result in a new element with an atomic mass of 232 - 3(4) = 220, and a new atomic number of 90 - 3(2) = 84.
Using the periodic table, we find the element with this atomic number is polonium (Po).
Compare your answer with the correct one above
Consider the following isotope:

What is the identity of the product after the following series of decay reactions?
alpha decay, alpha decay, electron emission, positron emission, positron emission
Consider the following isotope:
What is the identity of the product after the following series of decay reactions?
alpha decay, alpha decay, electron emission, positron emission, positron emission
In alpha decay, a helium nucleus is emitted, and thus the isotope loses 2 protons and 2 neutrons.
In electron emission, a neutron in the nucleus is converted into a proton and an emitted electron.
In positron emission, a proton in the nucleus is converted into a neutron and an emitted positron.
The given isotope will lose 4 protons and 4 neutrons via alpha emission, gain 1 proton and lose 1 neutron via electron emission, and lose 2 protons and gain 2 neutrons via positron emission. The result is a loss of 5 protons and 8 mass units.
Accounting for the changes in atomic mass and number, we find that the final element is 141-praseodymium.
In alpha decay, a helium nucleus is emitted, and thus the isotope loses 2 protons and 2 neutrons.
In electron emission, a neutron in the nucleus is converted into a proton and an emitted electron.
In positron emission, a proton in the nucleus is converted into a neutron and an emitted positron.
The given isotope will lose 4 protons and 4 neutrons via alpha emission, gain 1 proton and lose 1 neutron via electron emission, and lose 2 protons and gain 2 neutrons via positron emission. The result is a loss of 5 protons and 8 mass units.
Accounting for the changes in atomic mass and number, we find that the final element is 141-praseodymium.
Compare your answer with the correct one above
What hybrid orbitals are found in a molecule of water?
What hybrid orbitals are found in a molecule of water?
The hybrid orbitals for a molecule are determined by the number of atoms and lone pairs attached to the central atom. These two numbers are added to each other in order to determine the hybridization of the bonds. If there are two atoms or lone pairs attached to the central atom, it will exhibit sp hybridization. If there are three total atoms or lone pairs attached to the central atom, it will display sp2 hybridization. Water has two hydrogens and two lone pairs attached. This means that water has sp3 hybridization.
The hybrid orbitals for a molecule are determined by the number of atoms and lone pairs attached to the central atom. These two numbers are added to each other in order to determine the hybridization of the bonds. If there are two atoms or lone pairs attached to the central atom, it will exhibit sp hybridization. If there are three total atoms or lone pairs attached to the central atom, it will display sp2 hybridization. Water has two hydrogens and two lone pairs attached. This means that water has sp3 hybridization.
Compare your answer with the correct one above

What is the hybridization of the indicated carbon in the given molecule?

What is the hybridization of the indicated carbon in the given molecule?
The correct answer is sp2. First knock out any answers that aren't actual orbitals (for example sppp is not the proper notation so you could knock that out as an answer choice). After doing this you can tackle the problem using the following summary:
Electrons are contained within orbitals that carry different names (s, p, d, f) based on how far they are from the nucleus. The further these atomic orbitals are away from the nucleus, the higher in energy they are. When two atoms come together to form a covalent bond, their outer most orbitals overlap and the electrons within them are shared. Two s atomic orbitals overlapping would be lower energy (and thus more stable) than say if an s and a p orbital or two p orbitals overlapped.
The geometry associated with compounds and the strengths of each bond would then be varied based on which atomic orbitals decided to overlap, but in simple molecules like methane  the bond strengths are all equivalent and the molecular geometry is quite uniform/precise. It was theorized that all the orbitals involved in bonding might merge and form new shapes called hybrid orbitals. This allowed all the hybrid orbitals to obtain the same energy level as seen in nature. So if an s atomic orbital and a p atomic orbital combined, they would form an sp hybrid orbital with combined properties of both those atomic orbitals.
the bond strengths are all equivalent and the molecular geometry is quite uniform/precise. It was theorized that all the orbitals involved in bonding might merge and form new shapes called hybrid orbitals. This allowed all the hybrid orbitals to obtain the same energy level as seen in nature. So if an s atomic orbital and a p atomic orbital combined, they would form an sp hybrid orbital with combined properties of both those atomic orbitals.
When determining how many atomic orbitals are being used in covalent bonds for an atom within a molecule, you first count how many sigma (single) bonds there are and ignore any pi (double) bonds. If there are 3 sigma bonds, that means you have s + p + p atomic orbitals combining to form 3 sp3 hybrid orbitals (and so the hybridization of that atom would be sp3). We ignore pi (double) bonds because they are formed by the overlap to 2 p atomic orbitals. Also don’t forget to count invisible hydrogens that are not always drawn in.
The correct answer is sp2. First knock out any answers that aren't actual orbitals (for example sppp is not the proper notation so you could knock that out as an answer choice). After doing this you can tackle the problem using the following summary:
Electrons are contained within orbitals that carry different names (s, p, d, f) based on how far they are from the nucleus. The further these atomic orbitals are away from the nucleus, the higher in energy they are. When two atoms come together to form a covalent bond, their outer most orbitals overlap and the electrons within them are shared. Two s atomic orbitals overlapping would be lower energy (and thus more stable) than say if an s and a p orbital or two p orbitals overlapped.
The geometry associated with compounds and the strengths of each bond would then be varied based on which atomic orbitals decided to overlap, but in simple molecules like methane 
When determining how many atomic orbitals are being used in covalent bonds for an atom within a molecule, you first count how many sigma (single) bonds there are and ignore any pi (double) bonds. If there are 3 sigma bonds, that means you have s + p + p atomic orbitals combining to form 3 sp3 hybrid orbitals (and so the hybridization of that atom would be sp3). We ignore pi (double) bonds because they are formed by the overlap to 2 p atomic orbitals. Also don’t forget to count invisible hydrogens that are not always drawn in.
Compare your answer with the correct one above

What is the hybridization of the indicated carbon in the given molecule?

What is the hybridization of the indicated carbon in the given molecule?
The correct answer is sp3. First knock out any answers that aren't actual orbitals (for example spf skipped the d orbital entirely). After doing this, tackle the problem using the following guidelines:
Electrons are contained within orbitals that carry different names (s, p, d, f) based on how far they are from the nucleus. The further these atomic orbitals are away from the nucleus, the higher in energy they are. When two atoms come together to form a covalent bond, their outer most orbitals overlap and the electrons within them are shared. Two s atomic orbitals overlapping would be lower energy (and thus more stable) than say if an s and a p orbital or two p orbitals overlapped.
The geometry associated with compounds and the strengths of each bond would then be varied based on which atomic orbitals decided to overlap, but in simple molecules like methane  the bond strengths are all equivalent and the molecular geometry is quite uniform/precise. It was theorized that all the orbitals involved in bonding might merge and form new shapes called hybrid orbitals. This allowed all the hybrid orbitals to obtain the same energy level as seen in nature. So if an s atomic orbital and a p atomic orbital combined, they would form an sp hybrid orbital with combined properties of both those atomic orbitals.
the bond strengths are all equivalent and the molecular geometry is quite uniform/precise. It was theorized that all the orbitals involved in bonding might merge and form new shapes called hybrid orbitals. This allowed all the hybrid orbitals to obtain the same energy level as seen in nature. So if an s atomic orbital and a p atomic orbital combined, they would form an sp hybrid orbital with combined properties of both those atomic orbitals.
When determining how many atomic orbitals are being used in covalent bonds for an atom within a molecule, you first count how many sigma (single) bonds there are and ignore any pi (double) bonds. If there are 3 sigma bonds, that means you have s + p + p atomic orbitals combining to form 3 sp3 hybrid orbitals (and so the hybridization of that atom would be sp3). We ignore pi (double) bonds because they are formed by the overlap to 2 p atomic orbitals. Also don’t forget to count invisible hydrogens that are not always drawn in.
The correct answer is sp3. First knock out any answers that aren't actual orbitals (for example spf skipped the d orbital entirely). After doing this, tackle the problem using the following guidelines:
Electrons are contained within orbitals that carry different names (s, p, d, f) based on how far they are from the nucleus. The further these atomic orbitals are away from the nucleus, the higher in energy they are. When two atoms come together to form a covalent bond, their outer most orbitals overlap and the electrons within them are shared. Two s atomic orbitals overlapping would be lower energy (and thus more stable) than say if an s and a p orbital or two p orbitals overlapped.
The geometry associated with compounds and the strengths of each bond would then be varied based on which atomic orbitals decided to overlap, but in simple molecules like methane 
When determining how many atomic orbitals are being used in covalent bonds for an atom within a molecule, you first count how many sigma (single) bonds there are and ignore any pi (double) bonds. If there are 3 sigma bonds, that means you have s + p + p atomic orbitals combining to form 3 sp3 hybrid orbitals (and so the hybridization of that atom would be sp3). We ignore pi (double) bonds because they are formed by the overlap to 2 p atomic orbitals. Also don’t forget to count invisible hydrogens that are not always drawn in.
Compare your answer with the correct one above