Equilibrium Constant - GRE Subject Test: Chemistry
Card 0 of 1
Question
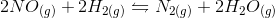
In the above reaction, by what factor would the reaction quotient change if the concentration of 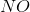 were doubled?
were doubled?
In the above reaction, by what factor would the reaction quotient change if the concentration of 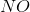
Answer
For a general chemical equation 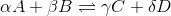 , where A, B, C, and D are elements and the Greek letters are their coefficients, we have the reaction quotient equation:
, where A, B, C, and D are elements and the Greek letters are their coefficients, we have the reaction quotient equation:
![Q=\frac{[C]^{\gamma}[D]^{\delta}}{[A]^{\alpha}[B]^{\beta}}](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/668140/gif.latex)
We can find the reaction quotient equation for our reaction by substituting the variables.

![Q=\frac{[N_{2}][H_{2}O]^{2}}{[NO]^{2}{[H_{2}]}^{2}}](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/668141/gif.latex)
Notice that the concentration of  is in the denominator and is squared, so doubling the concentration of
is in the denominator and is squared, so doubling the concentration of  changes the reaction quotient by a factor of one-fourth.
changes the reaction quotient by a factor of one-fourth.
![Q_2=\frac{[N_{2}][H_{2}O]^{2}}{[2NO]^{2}{[H_{2}]}^{2}}=\frac{[N_{2}][H_{2}O]^{2}}{4[NO]^{2}{[H_{2}]}^{2}}](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/668142/gif.latex)

For a general chemical equation 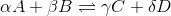
We can find the reaction quotient equation for our reaction by substituting the variables.
Notice that the concentration of 

Compare your answer with the correct one above
Tap the card to reveal the answer