Analyzing Data - GRE Subject Test: Chemistry
Card 0 of 19
Michael's scale measures the mass of objects as consistently  less than their actual mass. How would you describe the scale?
less than their actual mass. How would you describe the scale?
Michael's scale measures the mass of objects as consistently 
Precision measures is how consistently a device records the same answer. In this case, Michael's scale is ALWAYS  short. Even though it displays the wrong value, it is consistent. That means it is precise. Measuring a
short. Even though it displays the wrong value, it is consistent. That means it is precise. Measuring a  object will always display a mass of
object will always display a mass of  ; the results are easily reproduced.
; the results are easily reproduced.
Accuracy is how well a device measures something against its accepted value. In this case, Michael's scale is not accurate because it is always off by  .
.
Precision measures is how consistently a device records the same answer. In this case, Michael's scale is ALWAYS 


Accuracy is how well a device measures something against its accepted value. In this case, Michael's scale is not accurate because it is always off by 
Compare your answer with the correct one above
Michael buys several bags of balloons. On the package, it says that each bag has 100 balloons. He opens the bags and only one of them has 100 balloons inside; the other bags either have too many or too few.
How would you describe the bag of balloons with 100 balloons inside?
Michael buys several bags of balloons. On the package, it says that each bag has 100 balloons. He opens the bags and only one of them has 100 balloons inside; the other bags either have too many or too few.
How would you describe the bag of balloons with 100 balloons inside?
This bag is accurate because it provided the correct number of balloons, however, the process is not precise as the results were clearly not repeatable.
Accuracy deals with how close the measurement got to the accepted measurement. Precision deals with how consistent the measurement is. The bag with 100 balloons inside matched the claim made on the bag, meaning it was accurate. It was not precise because the other measurements show that the number of balloons is variable.
This bag is accurate because it provided the correct number of balloons, however, the process is not precise as the results were clearly not repeatable.
Accuracy deals with how close the measurement got to the accepted measurement. Precision deals with how consistent the measurement is. The bag with 100 balloons inside matched the claim made on the bag, meaning it was accurate. It was not precise because the other measurements show that the number of balloons is variable.
Compare your answer with the correct one above
An brand of fruit snacks claims that each bag of fruit snacks has a mass of  . After weighing three bags, Wally observes the masses to be
. After weighing three bags, Wally observes the masses to be  ,
,  , and
, and  .
.
How can Wally describe the accuracy and precision of the first bag he measured?
An brand of fruit snacks claims that each bag of fruit snacks has a mass of 



How can Wally describe the accuracy and precision of the first bag he measured?
The claim for the mass of the first bag is accurate; the brand says there should be  in each bag and there was
in each bag and there was  in the first bag.
in the first bag.
The claim on the first bag is not precise, as the results are not replicated universally throughout the experiment. The masses of the bags fluctuate, with the average of all three bags equal to  .
.
The claim for the mass of the first bag is accurate; the brand says there should be 

The claim on the first bag is not precise, as the results are not replicated universally throughout the experiment. The masses of the bags fluctuate, with the average of all three bags equal to 
Compare your answer with the correct one above
A bowman is shooting arrows at a target. Which of the following demonstrates high accuracy but low precision?
A bowman is shooting arrows at a target. Which of the following demonstrates high accuracy but low precision?
Accuracy is measured as the degree of closeness to the actual measurement. In our case, accurate shots will hit the bullseye. Precision is measured as the degree of closeness of one measurement to the next. In our case, precise shots will be clustered together.
To get high accuracy but low precision, measurements must center around the target value but be variable. The bowman's arrows will not be clustered (low precision), but will be accurately distributed around the bullseye. If all the shots were averaged, the bullseye would be at the center.
Accuracy is measured as the degree of closeness to the actual measurement. In our case, accurate shots will hit the bullseye. Precision is measured as the degree of closeness of one measurement to the next. In our case, precise shots will be clustered together.
To get high accuracy but low precision, measurements must center around the target value but be variable. The bowman's arrows will not be clustered (low precision), but will be accurately distributed around the bullseye. If all the shots were averaged, the bullseye would be at the center.
Compare your answer with the correct one above
Which of these is an example of high accuracy?
Which of these is an example of high accuracy?
Accuracy is the measure of difference between a calculated value and the true value of a measurement. High accuracy demands that the experimental result be equal to the theoretical result.
In contrast, precision is a measure of reproducibility. If multiple trials produce the same result each time with minimal deviation, then the experiment has high precision. This is true even if the results are not true to the theoretical predictions; an experiment can have high precision with low accuracy.
An archer hitting a bulls-eye is an example of high accuracy, while an archer hitting the same spot on the bulls-eye three times would be an example of high precision.
Accuracy is the measure of difference between a calculated value and the true value of a measurement. High accuracy demands that the experimental result be equal to the theoretical result.
In contrast, precision is a measure of reproducibility. If multiple trials produce the same result each time with minimal deviation, then the experiment has high precision. This is true even if the results are not true to the theoretical predictions; an experiment can have high precision with low accuracy.
An archer hitting a bulls-eye is an example of high accuracy, while an archer hitting the same spot on the bulls-eye three times would be an example of high precision.
Compare your answer with the correct one above
Which of these is an example of high precision?
Which of these is an example of high precision?
Precision is a measure of reproducibility. If multiple trials produce the same result each time with minimal deviation, then the experiment has high precision. This is true even if the results are not true to the theoretical predictions; an experiment can have high precision with low accuracy.
In contrast, accuracy is the measure of difference between a calculated value and the true value of a measurement. High accuracy demands that the experimental result be equal to the theoretical result.
An archer hitting a bulls-eye is an example of high accuracy, while an archer hitting the same spot on the bulls-eye three times would be an example of high precision.
Precision is a measure of reproducibility. If multiple trials produce the same result each time with minimal deviation, then the experiment has high precision. This is true even if the results are not true to the theoretical predictions; an experiment can have high precision with low accuracy.
In contrast, accuracy is the measure of difference between a calculated value and the true value of a measurement. High accuracy demands that the experimental result be equal to the theoretical result.
An archer hitting a bulls-eye is an example of high accuracy, while an archer hitting the same spot on the bulls-eye three times would be an example of high precision.
Compare your answer with the correct one above
What is the molar concentration of a 2% by mass  solution with a density of
solution with a density of  ?
?
What is the molar concentration of a 2% by mass 

By definition, 2% by mass means 2 grams of  in every 100 grams of solution. In order to solve this problem, we may assume we are dealing with 100 grams of solution. We can use the density as a conversion factor to determine the volume of solution we are dealing with:
in every 100 grams of solution. In order to solve this problem, we may assume we are dealing with 100 grams of solution. We can use the density as a conversion factor to determine the volume of solution we are dealing with:


In order to calculate the number of moles of  , we need to convert 2 grams of
, we need to convert 2 grams of  to moles using it's molecular weight. This is because we are assuming we are dealing 100 grams of a 2%
to moles using it's molecular weight. This is because we are assuming we are dealing 100 grams of a 2%  solution.
solution.

In order to calculate molarity, we need to plug the moles and volumes calculated into the following equation:

By definition, 2% by mass means 2 grams of 
In order to calculate the number of moles of 


In order to calculate molarity, we need to plug the moles and volumes calculated into the following equation:
Compare your answer with the correct one above

Using the calibration curve given, if given the absorbance of 1.51, what would be the concentration?

Using the calibration curve given, if given the absorbance of 1.51, what would be the concentration?
A calibration curve is a graph that gives the relationship between a measured quantity and an analytical signal. In this question the analytical signal is absorbance and the measured quantity is the concentration. To answer this problem, simply determine where the absorbance value 1.5 is on the y-axis and find what point the x-axis crosses this absorbance.
A calibration curve is a graph that gives the relationship between a measured quantity and an analytical signal. In this question the analytical signal is absorbance and the measured quantity is the concentration. To answer this problem, simply determine where the absorbance value 1.5 is on the y-axis and find what point the x-axis crosses this absorbance.
Compare your answer with the correct one above
A researcher performs a Bradford assay to determine the quantity of an unknown protein in his sample. The standard protein returns absorbance values of 0.101, 0.204, 0.302, 0.405 for the respective quantities of 10ug, 20ug, 30ug, and 40ug of protein. The unknown sample returns an absorbance value of 0.265. What is the quantity of protein in the unknown sample?
A researcher performs a Bradford assay to determine the quantity of an unknown protein in his sample. The standard protein returns absorbance values of 0.101, 0.204, 0.302, 0.405 for the respective quantities of 10ug, 20ug, 30ug, and 40ug of protein. The unknown sample returns an absorbance value of 0.265. What is the quantity of protein in the unknown sample?
For this problem we need to determine the equation of our standard curve. This can be done by creating a graph from the data points and determining the slope.

Assuming that a sample of zero concentration will also have zero absorbance, we can find the equation for the line generated by finding the slope.


Pick two points on the line to find the slope. We will use (0,0) and (40,0.405).


Use this equation and the absorbance given in the question to find the concentration of the unknown sample.


For this problem we need to determine the equation of our standard curve. This can be done by creating a graph from the data points and determining the slope.

Assuming that a sample of zero concentration will also have zero absorbance, we can find the equation for the line generated by finding the slope.
Pick two points on the line to find the slope. We will use (0,0) and (40,0.405).
Use this equation and the absorbance given in the question to find the concentration of the unknown sample.
Compare your answer with the correct one above
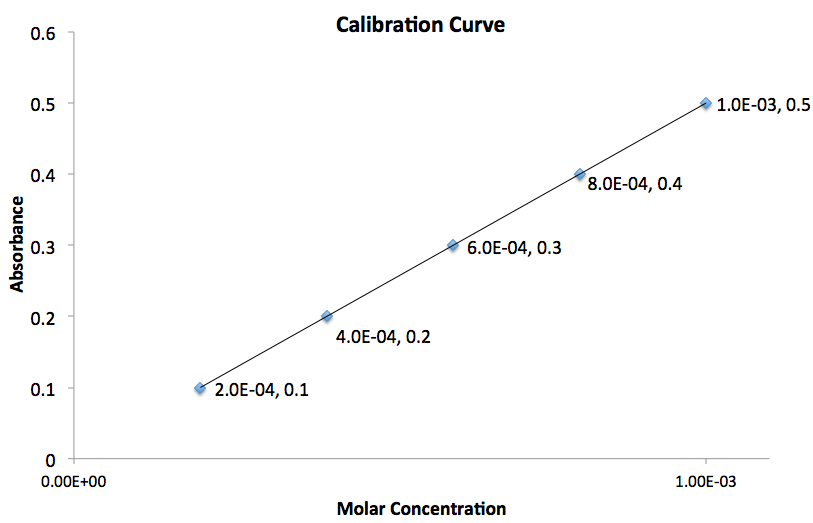
Using the graph given, what is the value for the slope of the line?

Using the graph given, what is the value for the slope of the line?
The slope of the line,  , can be calculated by the following equation:
, can be calculated by the following equation:

The subscripts are simply the points you chose in the order you arbitrarily chose.The most important thing to do is plug the values into equation in the order you chose them.
To determine the slope of the line we need to use two points on the graph. Assuming the two points chosen correspond to  and
and  , plugging these values into the equation gives:
, plugging these values into the equation gives:

Therefore the slope of the line is equal to 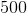
The slope of the line, 
The subscripts are simply the points you chose in the order you arbitrarily chose.The most important thing to do is plug the values into equation in the order you chose them.
To determine the slope of the line we need to use two points on the graph. Assuming the two points chosen correspond to 

Therefore the slope of the line is equal to
Compare your answer with the correct one above
Robert conducted an experiment in which he investigated how much water a paper towel could absorb. Initially, Robert found that one paper towel can absorb 12.8g of water. Later he found that his scale was not calibrated, so he had to repeat the experiment. After repeating the experiment with a new scale, Robert found that one paper towel can actually absorb 32.9g of water. What is the approximate percent error between the findings of the first and second experiments?
Robert conducted an experiment in which he investigated how much water a paper towel could absorb. Initially, Robert found that one paper towel can absorb 12.8g of water. Later he found that his scale was not calibrated, so he had to repeat the experiment. After repeating the experiment with a new scale, Robert found that one paper towel can actually absorb 32.9g of water. What is the approximate percent error between the findings of the first and second experiments?
The formula for percent error is:

In this case, the measured value is 12.8g and the accepted value is 32.9g.

The formula for percent error is:
In this case, the measured value is 12.8g and the accepted value is 32.9g.
Compare your answer with the correct one above
A reaction between one mole of sodium and one mole of chloride should yield 42 grams of sodium chloride. In your experiment, the actual yield is 32.73 grams. Calculate the percent error of your experiment.
A reaction between one mole of sodium and one mole of chloride should yield 42 grams of sodium chloride. In your experiment, the actual yield is 32.73 grams. Calculate the percent error of your experiment.
To find percent error we need to use the following equation:

Plug in 42 for the theoretical yield and 32.73 for the actual yield and solve accordingly.

To find percent error we need to use the following equation:
Plug in 42 for the theoretical yield and 32.73 for the actual yield and solve accordingly.
Compare your answer with the correct one above
In the following reaction, eight moles of sodium hydroxide is broken down into four moles of sodium oxide and four moles of water. What is the percent error if your experiment yields 195 grams of sodium oxide?

In the following reaction, eight moles of sodium hydroxide is broken down into four moles of sodium oxide and four moles of water. What is the percent error if your experiment yields 195 grams of sodium oxide?
To find the percent error we need to use the following equation:

But in order to do this, we first have to convert moles of sodium oxide into grams:

This gives us a theoretical yield of 248g, which we plug in with our 195g actual yield.

To find the percent error we need to use the following equation:
But in order to do this, we first have to convert moles of sodium oxide into grams:
This gives us a theoretical yield of 248g, which we plug in with our 195g actual yield.
Compare your answer with the correct one above
If a given sample of silver and flourine ideally combine to form 0.6mol of AgF, what is the percent error if the actual yield is 43 grams?
If a given sample of silver and flourine ideally combine to form 0.6mol of AgF, what is the percent error if the actual yield is 43 grams?
Our first step to complete this problem is to convert moles AgF into grams:

This gives us a theoretical yield of 76.2 grams. We can find the percent error by plugging in 76.2g for our theoretical yield and 43g for the actual yield in the following equation:


Our first step to complete this problem is to convert moles AgF into grams:
This gives us a theoretical yield of 76.2 grams. We can find the percent error by plugging in 76.2g for our theoretical yield and 43g for the actual yield in the following equation:
Compare your answer with the correct one above

Determine the percentage yield if the expected yield of  in the above reaction is 40.911 grams but the actual yield is 35.322 grams.
in the above reaction is 40.911 grams but the actual yield is 35.322 grams.

Determine the percentage yield if the expected yield of 
In chemistry, percentage yield is a term used to quantify the efficiency of a chemical reaction. In order to calculate this quantity, the value for the actual yield and theoretical yield is needed. The actual yield is the amount of product obtained during a chemical reaction. The theoretical yield is the maximum amount of product possible to be obtain by a chemical reaction which is based on the amount of reactants used. Below is the formula needed to calculate the percentage yield.


In chemistry, percentage yield is a term used to quantify the efficiency of a chemical reaction. In order to calculate this quantity, the value for the actual yield and theoretical yield is needed. The actual yield is the amount of product obtained during a chemical reaction. The theoretical yield is the maximum amount of product possible to be obtain by a chemical reaction which is based on the amount of reactants used. Below is the formula needed to calculate the percentage yield.
Compare your answer with the correct one above

0.0031 moles of  was reacted with 0.0071 moles of
was reacted with 0.0071 moles of  giving an actual yield of 0.1733 grams of
giving an actual yield of 0.1733 grams of  . What is the percent yield of this reaction?
. What is the percent yield of this reaction?

0.0031 moles of 


 is the limiting reactant because based on the equation,
is the limiting reactant because based on the equation,  and
and  react in a 1:1 mole ratio. However, there is 0.0040 moles more of
react in a 1:1 mole ratio. However, there is 0.0040 moles more of  than
than  present. Therefore the amount of
present. Therefore the amount of  produced is limited by the amount of
produced is limited by the amount of  present in the reaction. Based on the equation, for every 1 mole of
present in the reaction. Based on the equation, for every 1 mole of  reacted, 1 mole of
reacted, 1 mole of  was produced:
was produced:

Therefore,












Therefore,
Compare your answer with the correct one above

Determine the percentage yield if the expected yield of  in the above reaction is 1.2124 grams but the actual yield is 0.9749 grams.
in the above reaction is 1.2124 grams but the actual yield is 0.9749 grams.

Determine the percentage yield if the expected yield of 
In chemistry, percentage yield is a term used to quantify the efficiency of a chemical reaction. In order to calculate this quantity, the value for the actual yield and theoretical yield is needed. The actual yield is the amount of product obtained during a chemical reaction. The theoretical yield is the maximum amount of product possible to be obtain by a chemical reaction which is based on the amount of reactants used. Below is the formula needed to calculate the percentage yield.


In chemistry, percentage yield is a term used to quantify the efficiency of a chemical reaction. In order to calculate this quantity, the value for the actual yield and theoretical yield is needed. The actual yield is the amount of product obtained during a chemical reaction. The theoretical yield is the maximum amount of product possible to be obtain by a chemical reaction which is based on the amount of reactants used. Below is the formula needed to calculate the percentage yield.
Compare your answer with the correct one above
Robert's work schedule for next week will be released today. Robert will work either 45, 40, 25, or 12 hours. The probabilities for each possibility are listed below:
45 hours: 0.3
40 hours: 0.2
25 hours: 0.4
12 hours: 0.1
What is the standard deviation of the possible outcomes?
Robert's work schedule for next week will be released today. Robert will work either 45, 40, 25, or 12 hours. The probabilities for each possibility are listed below:
45 hours: 0.3
40 hours: 0.2
25 hours: 0.4
12 hours: 0.1
What is the standard deviation of the possible outcomes?
There are four steps to finding the standard deviation of random variables. First, calculate the mean of the random variables. Second, for each value in the group (45, 40, 25, and 12), subtract the mean from each and multiply the result by the probability of that outcome occurring. Third, add the four results together. Fourth, find the square root of the result.






There are four steps to finding the standard deviation of random variables. First, calculate the mean of the random variables. Second, for each value in the group (45, 40, 25, and 12), subtract the mean from each and multiply the result by the probability of that outcome occurring. Third, add the four results together. Fourth, find the square root of the result.
Compare your answer with the correct one above
We have two independent, normally distributed random variables  and
and  such that
such that  has mean
has mean  and variance
and variance  and
and  has mean
has mean  and variance
and variance  . What is the probability distribution of the difference of the random variables,
. What is the probability distribution of the difference of the random variables,  ?
?
We have two independent, normally distributed random variables 








The mean for any set of random variables is additive in the sense that

The difference is also additive, so we have

This means the mean of  is
is  .
.
The variance is additive when the random variables are independent, which they are in this case. But it's additive in the sense that for any real numbers  (even when negative), we have
(even when negative), we have
 .
.
So for this difference, we have

 .
.
So the mean and variance are  and
and  , respectively. In addition to that,
, respectively. In addition to that,  is normally distributed because the sum or difference of any set of independent normal random variables is also normally distributed.
is normally distributed because the sum or difference of any set of independent normal random variables is also normally distributed.
The mean for any set of random variables is additive in the sense that
The difference is also additive, so we have
This means the mean of 

The variance is additive when the random variables are independent, which they are in this case. But it's additive in the sense that for any real numbers 

So for this difference, we have

So the mean and variance are 


Compare your answer with the correct one above