Titrations
Practice Questions
GRE Subject Test: Chemistry › Titrations
Considering the given chemical reaction, determine the number of moles of 

Which of the following is an indication of when a reaction has reached the equivalence point.
A 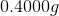




Based on the pH titration curve provided, how many acidic protons are in this acid?
Considering the given neutralization reaction, what is the concentration of the sodium chloride solution if it takes 20.00mL of 0.045M 

A vinegar sample was determined by a titration with 


Which of the following pH values is an acceptable equivalence point for a weak base being titrated by a strong acid?
A weak acid is slowly titrated with a strong base. Where on the titration curve would the solution be the most well-buffered?
Considering the given chemical reaction, determine the concentration of 

What volume in liters of 





