Making Solutions - GED Science
Card 0 of 1
Question
A student is asked to make  of
of  NaCl solution. The student is given a stock solution of
NaCl solution. The student is given a stock solution of 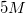 NaCl. What volumes of stock NaCl solution and pure water could be used to make the desired final solution?
NaCl. What volumes of stock NaCl solution and pure water could be used to make the desired final solution?
A student is asked to make 

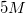
Answer
Convert liters to mL:

This is the TOTAL volume of the solution. Next, determine how much NaCl to add.








Therefore,  of stock NaCl must be added. Subtract this volume from
of stock NaCl must be added. Subtract this volume from 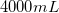 to determine the amount of water used in the final solution.
to determine the amount of water used in the final solution.

Convert liters to mL:
This is the TOTAL volume of the solution. Next, determine how much NaCl to add.
Therefore, 
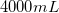
Compare your answer with the correct one above
Tap the card to reveal the answer