Solutions
Practice Questions
GED Science › Solutions
Questions
4
1
A student is asked to make 

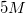
2
A student is asked to make 

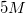
3
6g of sugar is dissolved in 250mL of water. What is the percent concentration of sugar in mass per unit volume?
4
6g of sugar is dissolved in 250mL of water. What is the percent concentration of sugar in mass per unit volume?