Thermochemistry and Changes of State - College Chemistry
Card 0 of 15
Which of the following is true of a closed system?
Which of the following is true of a closed system?
A closed system allows for the exchange of energy between the system and its surroundings, but does not allow the exchange of matter. This is the definition of a closed system. An open system allows for the exchange of both matter and energy between the system and its surroundings. An isolated system on the other hand does not allow the exchange of either matter or energy between the system and its surroundings.
A closed system allows for the exchange of energy between the system and its surroundings, but does not allow the exchange of matter. This is the definition of a closed system. An open system allows for the exchange of both matter and energy between the system and its surroundings. An isolated system on the other hand does not allow the exchange of either matter or energy between the system and its surroundings.
Compare your answer with the correct one above
When  of liquid octane
of liquid octane  undergoes combustion in a bomb calorimeter, the temperature increases from
undergoes combustion in a bomb calorimeter, the temperature increases from  degrees Celsius to
degrees Celsius to  degrees Celsius. The heat capacity for the bomb calorimeter is
degrees Celsius. The heat capacity for the bomb calorimeter is  . Find the
. Find the  for the combustion of octane in
for the combustion of octane in  .
.
When 






Recall the following equation:

Now, since the bomb calorimeter keeps the volume constant, we know the following relationship:

Thus, we can then write the following equation for  :
:

Start by finding  :
:

From this, we know that 
Now, find  .
.

Your answer must have  significant figures.
significant figures.

Recall the following equation:
Now, since the bomb calorimeter keeps the volume constant, we know the following relationship:
Thus, we can then write the following equation for 
Start by finding 
From this, we know that
Now, find 
Your answer must have 
Compare your answer with the correct one above
How much heat energy is needed to raise the temperature of  of copper from
of copper from  to
to  ? The specific heat capacity of copper is
? The specific heat capacity of copper is  .
.
How much heat energy is needed to raise the temperature of 



In this question, we're given the mass of copper, along with its specific heat capacity, and we're asked to determine the amount of heat energy necessary to increase its temperature by a given amount.
To solve this problem, we'll need to make use of the following equation.

Since we know what the values are for the mass and specific heat, we'll need to figure out what the temperature will be. Since the Kelvin and Celsius temperature scales both change by the same amount and only differ at their zero point, we can take the difference of the temperatures in degrees Celsius and use that value (since it will be equivalent to the change in the Kelvin temperature as well).


Plugging this information into the first expression, we can solve for the amount of heat energy that will bring this mass of copper to the desired temperature.


In this question, we're given the mass of copper, along with its specific heat capacity, and we're asked to determine the amount of heat energy necessary to increase its temperature by a given amount.
To solve this problem, we'll need to make use of the following equation.
Since we know what the values are for the mass and specific heat, we'll need to figure out what the temperature will be. Since the Kelvin and Celsius temperature scales both change by the same amount and only differ at their zero point, we can take the difference of the temperatures in degrees Celsius and use that value (since it will be equivalent to the change in the Kelvin temperature as well).
Plugging this information into the first expression, we can solve for the amount of heat energy that will bring this mass of copper to the desired temperature.
Compare your answer with the correct one above
Ice cubes are dropped into a glass of water. You notice that the glass of water becomes colder and condensation appears. Later in the day, you notice the glass of water is now room temperature and there is no more condensation. Which of the following concepts describes this process?
Ice cubes are dropped into a glass of water. You notice that the glass of water becomes colder and condensation appears. Later in the day, you notice the glass of water is now room temperature and there is no more condensation. Which of the following concepts describes this process?
Thermal equilibrium: Thermal energy of the system is equal to thermal energy of the surroundings. Heat is defined as the energy transfer resulting from differences in thermal energy. Heat always flows from higher temperature to lower temperature. Heat transfer between a system and its surroundings stop when they reach thermal equilibrium, or when there is no difference in thermal energy. In this case, ice was dropped into the cup. Initially, the ice increases the temperature of the water, creating a cold glass with condensation. However, energy from the surroundings flow into the system (the glass of water) due to thermal difference and warm the glass of water until the two reach thermal equilibrium. At thermal equilibrium, there is no ice, no condensation, and the water temperature is room temperature.
Thermal equilibrium: Thermal energy of the system is equal to thermal energy of the surroundings. Heat is defined as the energy transfer resulting from differences in thermal energy. Heat always flows from higher temperature to lower temperature. Heat transfer between a system and its surroundings stop when they reach thermal equilibrium, or when there is no difference in thermal energy. In this case, ice was dropped into the cup. Initially, the ice increases the temperature of the water, creating a cold glass with condensation. However, energy from the surroundings flow into the system (the glass of water) due to thermal difference and warm the glass of water until the two reach thermal equilibrium. At thermal equilibrium, there is no ice, no condensation, and the water temperature is room temperature.
Compare your answer with the correct one above
Ice can be used to counter the effects of overeating. How many kilograms of ice at  would one have to eat in order to cancel the effect of eating
would one have to eat in order to cancel the effect of eating  ? Assume body temperature to be
? Assume body temperature to be  .
.

Specific heat of water = 
Specific heat of ice = 

Ice can be used to counter the effects of overeating. How many kilograms of ice at 


Specific heat of water =
Specific heat of ice =
Recall that the heat gained by ice must be equal to the heat from the food.
Start by calculating the heat from the food by converting the Calories into joules.

Next, calculate the heat gained by the ice. Take this in three steps:
1. Heating the ice from  to
to 


2. Ice melting.


3. Raising the temperature of water from  to
to  .
.


Set this value equal to the heat from the food and solve for the mass.


Convert to kilograms.

Recall that the heat gained by ice must be equal to the heat from the food.
Start by calculating the heat from the food by converting the Calories into joules.
Next, calculate the heat gained by the ice. Take this in three steps:
1. Heating the ice from 
2. Ice melting.
3. Raising the temperature of water from 

Set this value equal to the heat from the food and solve for the mass.
Convert to kilograms.
Compare your answer with the correct one above
Suppose that  of a certain compound is needed in order to lower the freezing point of
of a certain compound is needed in order to lower the freezing point of  of water by
of water by  . What is the molar mass of this compound?
. What is the molar mass of this compound?
Note: Liquid water has a density of  and a molal freezing-point depression constant of
and a molal freezing-point depression constant of  .
.
Suppose that 


Note: Liquid water has a density of 

In this question, we're told that a certain amount of a compound dissolved in water lowers the water's freezing point. We're asked to determine the molar mass of the compound.
First, we must recognize this as a freezing point depression problem. Recall that freezing point depression is one of the colligative properties associated with dissolving solute into a solvent such as water.
To begin, we need to use the equation for freezing point depression, which states that the change in freezing point is proportional to both the molality of the solution as well as the molal freezing point depression constant for the solvent in question (in this case, water).



The answer we have just calculated above is the molality, which is a different way of expressing concentration than molarity. Molality is expressed as the number of moles of solute per kilogram of solvent, whereas molarity is the number of moles of solute divided by the total volume of the solution.
The next thing we need to do is find out how many total moles of solute are present in the solvent water. To do this, we need to use the volume of water provided to us in the question, along with the density of water, to calculate the mass of water present. Together with the molality calculated above, this will allow us to know the total number of moles in solution.


Now that we know the total number of moles of solute (our compound) that exists in solution, we can use that information, together with the total mass of the compound given to us in the question stem, to calculate the compound's molar mass.

In this question, we're told that a certain amount of a compound dissolved in water lowers the water's freezing point. We're asked to determine the molar mass of the compound.
First, we must recognize this as a freezing point depression problem. Recall that freezing point depression is one of the colligative properties associated with dissolving solute into a solvent such as water.
To begin, we need to use the equation for freezing point depression, which states that the change in freezing point is proportional to both the molality of the solution as well as the molal freezing point depression constant for the solvent in question (in this case, water).
The answer we have just calculated above is the molality, which is a different way of expressing concentration than molarity. Molality is expressed as the number of moles of solute per kilogram of solvent, whereas molarity is the number of moles of solute divided by the total volume of the solution.
The next thing we need to do is find out how many total moles of solute are present in the solvent water. To do this, we need to use the volume of water provided to us in the question, along with the density of water, to calculate the mass of water present. Together with the molality calculated above, this will allow us to know the total number of moles in solution.
Now that we know the total number of moles of solute (our compound) that exists in solution, we can use that information, together with the total mass of the compound given to us in the question stem, to calculate the compound's molar mass.
Compare your answer with the correct one above
As heat energy is added to a cube of ice, it begins to melt into liquid water. Which of the following correctly identifies the change in temperature and the change in internal energy of the ice as heat is added to it?
As heat energy is added to a cube of ice, it begins to melt into liquid water. Which of the following correctly identifies the change in temperature and the change in internal energy of the ice as heat is added to it?
Remember that when any substance is undergoing a phase change, its temperature will remain constant. In other words, the energy being added is not increasing the average kinetic energy of any of the particles in the system.
Also, the internal energy will not remain constant. As heat energy is added, as in the question, the internal energy will necessarily increase. Even though the kinetic energy of the particles is not increasing, the potential energy is. This is because the intermolecular forces of attraction (mostly hydrogen bonds in this case) need to be broken apart. When energy is added, that raises the potential energy component of internal energy, despite the fact that kinetic energy (and thus, temperature) remains constant.
Remember that when any substance is undergoing a phase change, its temperature will remain constant. In other words, the energy being added is not increasing the average kinetic energy of any of the particles in the system.
Also, the internal energy will not remain constant. As heat energy is added, as in the question, the internal energy will necessarily increase. Even though the kinetic energy of the particles is not increasing, the potential energy is. This is because the intermolecular forces of attraction (mostly hydrogen bonds in this case) need to be broken apart. When energy is added, that raises the potential energy component of internal energy, despite the fact that kinetic energy (and thus, temperature) remains constant.
Compare your answer with the correct one above
Consider the typical phase diagram of a compound given below.
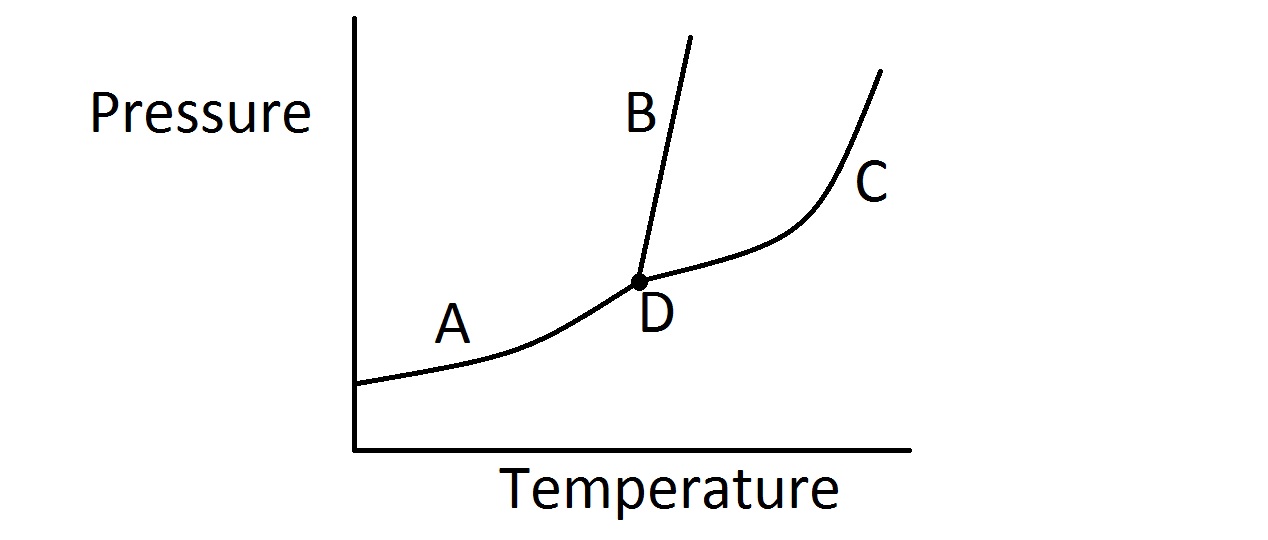
Which of the following lines or points on the diagram represents a situation in which the rate of vaporization of the compound is equal to its rate of condensation?
Consider the typical phase diagram of a compound given below.

Which of the following lines or points on the diagram represents a situation in which the rate of vaporization of the compound is equal to its rate of condensation?
In this question, we're presented with a phase diagram and are asked to determine where on the graph the rate of vaporization equals the rate of condensation.
First, it's important to realize that when the rate of vaporization and condensation are equal, we have an equilibrium of liquid and gas phases. In other words, for a given temperature and pressure, the rate at which the liquid evaporates into a gas is exactly equal to the rate at which the gas condenses into a liquid.
On a phase diagram, the area of the upper left portion of the diagram represents the solid state. The middle portion of the diagram represents the liquid state. The bottom and right most part of the diagram represents the gas phase.
Furthermore, each line on the diagram represents the specific combination of temperature and pressure in which a given compound will exist in equilibrium between two phases. The point where all three lines intersect, however, represents the triple point. This tells us the temperature and pressure in which the compound will exist in an equilibrium between all three states.
Because we are looking for the equilibrium line that represents equilibrium of vaporization and condensation, we want the line that separates the liquid portion of the diagram from the gas portion. Based on the identification of regions on the diagram discussed above, that would be line C as shown in the diagram. Line A represents equilibrium between solid and gas (sublimation rate = deposition rate). Line B represents equilibrium between solid and liquid (melting rate = freezing rate).
In this question, we're presented with a phase diagram and are asked to determine where on the graph the rate of vaporization equals the rate of condensation.
First, it's important to realize that when the rate of vaporization and condensation are equal, we have an equilibrium of liquid and gas phases. In other words, for a given temperature and pressure, the rate at which the liquid evaporates into a gas is exactly equal to the rate at which the gas condenses into a liquid.
On a phase diagram, the area of the upper left portion of the diagram represents the solid state. The middle portion of the diagram represents the liquid state. The bottom and right most part of the diagram represents the gas phase.
Furthermore, each line on the diagram represents the specific combination of temperature and pressure in which a given compound will exist in equilibrium between two phases. The point where all three lines intersect, however, represents the triple point. This tells us the temperature and pressure in which the compound will exist in an equilibrium between all three states.
Because we are looking for the equilibrium line that represents equilibrium of vaporization and condensation, we want the line that separates the liquid portion of the diagram from the gas portion. Based on the identification of regions on the diagram discussed above, that would be line C as shown in the diagram. Line A represents equilibrium between solid and gas (sublimation rate = deposition rate). Line B represents equilibrium between solid and liquid (melting rate = freezing rate).
Compare your answer with the correct one above
A  block of silver initially at
block of silver initially at  absorbs
absorbs  of heat. If the specific heat capacity of silver is
of heat. If the specific heat capacity of silver is  , in degrees Celsius, what is the final temperature of the block of silver?
, in degrees Celsius, what is the final temperature of the block of silver?
A 



Recall the equation that gives the relationship between change in temperature and amount of heat:
 ,
,
where  ,
,
 ,
,
 , and
, and

Since the question asks for the final temperature, re-arrange the equation to solve for  .
.



Substitute in the given values to solve for the final temperature.

Recall the equation that gives the relationship between change in temperature and amount of heat:

where 


Since the question asks for the final temperature, re-arrange the equation to solve for 
Substitute in the given values to solve for the final temperature.
Compare your answer with the correct one above
How much heat does it require to make a  block of lead initially at
block of lead initially at  go to
go to  ? The specific heat capacity of lead is
? The specific heat capacity of lead is  .
.
How much heat does it require to make a 



Recall the equation that gives the relationship between the change in temperature and the amount of heat:
 , where
, where

 ,
,
 , and
, and

Substitute in the given values to find how much heat is required to increase the temperature of the block of lead the specified amount.

Make sure to round the answer to three significant figures.

Recall the equation that gives the relationship between the change in temperature and the amount of heat:



Substitute in the given values to find how much heat is required to increase the temperature of the block of lead the specified amount.
Make sure to round the answer to three significant figures.
Compare your answer with the correct one above
How much heat is needed to raise the temperature of 10.0 g of water from 10.0 oC to 35.0 oC?
Specific heat capacity for water is 
How much heat is needed to raise the temperature of 10.0 g of water from 10.0 oC to 35.0 oC?
Specific heat capacity for water is
Recall the relationship between heat and specific heat capacity

Plug in known values and solve for Q

Recall the relationship between heat and specific heat capacity
Plug in known values and solve for Q
Compare your answer with the correct one above
An insulated container is filled with 50.0 g of water at 15.0 oC. 120.0 g of lead is heated to 100 oC and added to the insulated container. What is the final temperature of the system once it comes to equilibrium?
Specific heat of water is 
Specific heat of lead is 
An insulated container is filled with 50.0 g of water at 15.0 oC. 120.0 g of lead is heated to 100 oC and added to the insulated container. What is the final temperature of the system once it comes to equilibrium?
Specific heat of water is
Specific heat of lead is
Since Pb starts at a higher temperature than the water, we know that energy (in the form of heat) will be transferred from Pb to water. Due to the law of conservation of energy, the exact same amount of energy lost by Pb must be gained by water.

Recall that

Combining the two equations, we have



Combine like terms then solve for final temperature


Since Pb starts at a higher temperature than the water, we know that energy (in the form of heat) will be transferred from Pb to water. Due to the law of conservation of energy, the exact same amount of energy lost by Pb must be gained by water.
Recall that
Combining the two equations, we have
Combine like terms then solve for final temperature
Compare your answer with the correct one above
The specific heat capacity is defined as the amount of heat energy necessary to change a given amount of a substance by a certain temperature. Which of the following correctly expresses the units of specific heat capacity?
The specific heat capacity is defined as the amount of heat energy necessary to change a given amount of a substance by a certain temperature. Which of the following correctly expresses the units of specific heat capacity?
For this question, we're given a definition for the specific heat capacity of a substance and we're asked to identify the correct units for this term.
We can also recall the equation that relates all of these terms.

Rearranging this expression to isolate the term for the specific heat capacity gives us the following.

Next, we can recall what units would be appropriate to use for each of the following terms in the above expression. The  term represents heat energy added to or removed from the system, so this value would be in units of joules. Next, the
term represents heat energy added to or removed from the system, so this value would be in units of joules. Next, the  term represents the mass of the substance, so grams can be used for this term. Finally, the
term represents the mass of the substance, so grams can be used for this term. Finally, the  term represents the change in temperature of the substance, so we can use the absolute temperature in kelvins here.
term represents the change in temperature of the substance, so we can use the absolute temperature in kelvins here.
Putting all this together gives us the following.

For this question, we're given a definition for the specific heat capacity of a substance and we're asked to identify the correct units for this term.
We can also recall the equation that relates all of these terms.
Rearranging this expression to isolate the term for the specific heat capacity gives us the following.
Next, we can recall what units would be appropriate to use for each of the following terms in the above expression. The 


Putting all this together gives us the following.
Compare your answer with the correct one above
Use average bond enthalpies to estimate the enthalpy change, in kilojoules, of the combustion of one mole of hexane  to form liquid water and carbon dioxide gas.
to form liquid water and carbon dioxide gas.
Type of Bond Average bond enthalpy 
Carbon-hydrogen single bond 414 Carbon-oxygen double bond 736 Oxygen-oxygen double bond 498 Oxygen-hydrogen single bond 464 Carbon-carbon single bond 347
Use average bond enthalpies to estimate the enthalpy change, in kilojoules, of the combustion of one mole of hexane 
| Type of Bond | Average bond enthalpy  |
|---|---|
| Carbon-hydrogen single bond | 414 |
| Carbon-oxygen double bond | 736 |
| Oxygen-oxygen double bond | 498 |
| Oxygen-hydrogen single bond | 464 |
| Carbon-carbon single bond | 347 |
Start by writing the balanced equation for the combustion of hexane:

Recall the following equation:

Remember that energy is required to break bonds, so  should be positive. Energy is also released when bonds are made so
should be positive. Energy is also released when bonds are made so  should be negative.
should be negative.
Next, draw out the Lewis structures of each molecule to figure out the number of bonds made or broken.
For the hexane, there are 
 bonds that must be broken, and
bonds that must be broken, and 
 bonds that must be broken.
bonds that must be broken.

For each oxygen, there is only 1 oxygen double bond to break. However, we will need to multiply this number by its stoichiometric coefficient.

For each carbon dioxide, there are  double bonds between carbon and oxygen to break. For the given equation, there are
double bonds between carbon and oxygen to break. For the given equation, there are  total of these double bonds because we have
total of these double bonds because we have  moles of carbon dioxide.
moles of carbon dioxide.

For each water, there are  single bonds between hydrogen and oxygen. For the given equation, there are a total of
single bonds between hydrogen and oxygen. For the given equation, there are a total of  of these bonds because we have
of these bonds because we have  moles of water.
moles of water.
Now, use the given information regarding to average bond enthalpies to find the change in enthalpy for the reaction.


Start by writing the balanced equation for the combustion of hexane:
Recall the following equation:
Remember that energy is required to break bonds, so 

Next, draw out the Lewis structures of each molecule to figure out the number of bonds made or broken.
For the hexane, there are 




For each oxygen, there is only 1 oxygen double bond to break. However, we will need to multiply this number by its stoichiometric coefficient.

For each carbon dioxide, there are 



For each water, there are 


Now, use the given information regarding to average bond enthalpies to find the change in enthalpy for the reaction.
Compare your answer with the correct one above
Suppose a source of water is boiling. If the ambient pressure above the surface of the water were to increase, which of the following would happen to the boiling water?
Suppose a source of water is boiling. If the ambient pressure above the surface of the water were to increase, which of the following would happen to the boiling water?
For this question, we're told that water is boiling and that the pressure above the surface of the water increases. We're asked to find out what will happen to the water.
It's important to remember the definition of a boiling liquid; the vapor pressure of the liquid is equal to the atmospheric pressure above the solution. Thus, if the atmospheric pressure increases, the vapor pressure would then become lower than the atmospheric pressure. As a result, the water would cease to boil. Moreover, its boiling point would increase because a higher temperature would be necessary in order to raise the vapor pressure enough so that it becomes equal to atmospheric pressure.
For this question, we're told that water is boiling and that the pressure above the surface of the water increases. We're asked to find out what will happen to the water.
It's important to remember the definition of a boiling liquid; the vapor pressure of the liquid is equal to the atmospheric pressure above the solution. Thus, if the atmospheric pressure increases, the vapor pressure would then become lower than the atmospheric pressure. As a result, the water would cease to boil. Moreover, its boiling point would increase because a higher temperature would be necessary in order to raise the vapor pressure enough so that it becomes equal to atmospheric pressure.
Compare your answer with the correct one above