Gases - College Chemistry
Card 0 of 20
Per Graham's law of effusion, how does the molar mass relate to both the rate and time of effusion?
Per Graham's law of effusion, how does the molar mass relate to both the rate and time of effusion?

This equation explicitly shows how the rate of effusion is inversely proportional to the molar mass of a gas in a gaseous solution.
Because  , time relates to molar mass by:
, time relates to molar mass by:

Simplifying this equation, we see that:

As a result, time relates directly to molar mass
This equation explicitly shows how the rate of effusion is inversely proportional to the molar mass of a gas in a gaseous solution.
Because 
Simplifying this equation, we see that:
As a result, time relates directly to molar mass
Compare your answer with the correct one above
At the same temperature, an unknown gas effuses at a rate that is  times that of oxygen. Find the molar mass, in grams per mole, of the unknown gas.
times that of oxygen. Find the molar mass, in grams per mole, of the unknown gas.
At the same temperature, an unknown gas effuses at a rate that is 
Recall Graham's Law of Effusion for two gases, A and B:

From the equation, we know the following:

Thus, we can solve for the molar mass of the unknown gas. Let  be the molar mass of the unknown gas.
be the molar mass of the unknown gas.



Make sure that your answer has  significant figures.
significant figures.
Recall Graham's Law of Effusion for two gases, A and B:
From the equation, we know the following:
Thus, we can solve for the molar mass of the unknown gas. Let 
Make sure that your answer has 
Compare your answer with the correct one above
Which of the following is a true statement with regards to the relative effusion rates of oxygen and carbon dioxide?
Which of the following is a true statement with regards to the relative effusion rates of oxygen and carbon dioxide?
We're being asked to compare the effusion rates of oxygen and carbon dioxide.
Remember that effusion is the spontaneous movement of a gas through a small hole from one area to another. It's worth noting that at a given temperature, the average speed of all gas molecules in a system is used to calculate the average kinetic energy of the gas particles. This dependence of kinetic energy on temperature means that at a given temperature, any gas particle will have the same kinetic energy.
In this case, we can say that the kinetic energy of oxygen molecules in one system is equal to the kinetic energy of carbon dioxide molecules in another system. Furthermore, since mass is inversely proportional to velocity, identical kinetic energies would mean that as the mass of the gas particles in a system decreases, their velocity (and thus, effusion rates) would increase.
We can use this information to solve for the relative effusion rates between oxygen and carbon dioxide. By setting their kinetic energies equal to each other, we can derive an expression that relates their relative speeds to their relative masses.





Generally speaking, this expression shows how the velocity of any two gasses depends on their mass. In this case, the gasses are oxygen and carbon dioxide.
We can use the periodic table of the elements to find out the mass of each gas, and use that information to calculate the relative effusion rates.


This shows that oxygen will effuse at a rate that is about  faster than carbon dioxide.
faster than carbon dioxide.
We're being asked to compare the effusion rates of oxygen and carbon dioxide.
Remember that effusion is the spontaneous movement of a gas through a small hole from one area to another. It's worth noting that at a given temperature, the average speed of all gas molecules in a system is used to calculate the average kinetic energy of the gas particles. This dependence of kinetic energy on temperature means that at a given temperature, any gas particle will have the same kinetic energy.
In this case, we can say that the kinetic energy of oxygen molecules in one system is equal to the kinetic energy of carbon dioxide molecules in another system. Furthermore, since mass is inversely proportional to velocity, identical kinetic energies would mean that as the mass of the gas particles in a system decreases, their velocity (and thus, effusion rates) would increase.
We can use this information to solve for the relative effusion rates between oxygen and carbon dioxide. By setting their kinetic energies equal to each other, we can derive an expression that relates their relative speeds to their relative masses.
Generally speaking, this expression shows how the velocity of any two gasses depends on their mass. In this case, the gasses are oxygen and carbon dioxide.
We can use the periodic table of the elements to find out the mass of each gas, and use that information to calculate the relative effusion rates.
This shows that oxygen will effuse at a rate that is about 
Compare your answer with the correct one above
A sample of Ne(g) effusses through a tiny hole in 60.7 s. An unknown gas, under identical conditions, effusses in 45.6 s.
What is the molar mass of the unknown gas?
A sample of Ne(g) effusses through a tiny hole in 60.7 s. An unknown gas, under identical conditions, effusses in 45.6 s.
What is the molar mass of the unknown gas?
To solve this problem use Graham's Law of Effusion



By plugging in the values we can rewrite the equation as



To solve this problem use Graham's Law of Effusion
By plugging in the values we can rewrite the equation as
Compare your answer with the correct one above
Suppose that gas A effuses at a rate that is twice that of gas B. If the mass of gas A is halved and the mass of gas B is doubled, which of the following correctly describes the new relative effusion rates of these two gasses?
Suppose that gas A effuses at a rate that is twice that of gas B. If the mass of gas A is halved and the mass of gas B is doubled, which of the following correctly describes the new relative effusion rates of these two gasses?
For this question, we're given the relative effusion rates for two gasses. We're then told how the mass of each of these gasses is changed, and then we're asked to determine the new relative effusion rates of the two gasses.
First, we can recall the expression that describes the dependence of the effusion rates of two gasses on their mass. Since we're told that the rate of gas A is twice that of gas B, we can write the following expression.

Furthermore, since we're told that the mass of gas B is doubled and the mass of gas A is halved, we can determine how the rate will change.

Thus, we can see that the rate will change by a factor of two. Hence, the new rate will be  . Thus, gas A will now effuse at a rate
. Thus, gas A will now effuse at a rate  times that of gas B.
times that of gas B.
For this question, we're given the relative effusion rates for two gasses. We're then told how the mass of each of these gasses is changed, and then we're asked to determine the new relative effusion rates of the two gasses.
First, we can recall the expression that describes the dependence of the effusion rates of two gasses on their mass. Since we're told that the rate of gas A is twice that of gas B, we can write the following expression.
Furthermore, since we're told that the mass of gas B is doubled and the mass of gas A is halved, we can determine how the rate will change.
Thus, we can see that the rate will change by a factor of two. Hence, the new rate will be 

Compare your answer with the correct one above
A sample of gas at a constant temperature has an initial pressure of  at a pressure of
at a pressure of  . If the volume of gas is decreased to
. If the volume of gas is decreased to  , what is its pressure?
, what is its pressure?
A sample of gas at a constant temperature has an initial pressure of 


Since we are given the volume and the pressure of this sample of gas, we will need to use Boyle's Law, which states that the pressure and volume of a gas, at a constant temperature, are inversely related. As thus, we can then write the following equation:

Since all the answer choices are in units of atmospheres, we will need to convert the given units into atmospheres.

Plug in the given pressures and volume into the equation, and solve for  .
.


Since we are given the volume and the pressure of this sample of gas, we will need to use Boyle's Law, which states that the pressure and volume of a gas, at a constant temperature, are inversely related. As thus, we can then write the following equation:
Since all the answer choices are in units of atmospheres, we will need to convert the given units into atmospheres.
Plug in the given pressures and volume into the equation, and solve for 
Compare your answer with the correct one above
What is the pressure of a  cylinder filled with
cylinder filled with  moles of helium gas at
moles of helium gas at  ?
?
What is the pressure of a 


Recall the ideal gas law:
 ,
,
where  ,
,
 ,
,
 ,
,
 ,
,
and  .
.
Since the question asks for pressure, rearrange the equation to solve for  .
.

Plug in the given values. Remember that 

Recall the ideal gas law:

where 



and 
Since the question asks for pressure, rearrange the equation to solve for 
Plug in the given values. Remember that
Compare your answer with the correct one above
Consider the following chemical reaction:

How many grams of lithium is needed to react with  of nitrogen gas measured at
of nitrogen gas measured at  and
and  ?
?
Consider the following chemical reaction:
How many grams of lithium is needed to react with 


Start by finding the number of moles of nitrogen gas by using the ideal gas law:

Rearrange the equation to solve for the variable  :
:

In order to use the ideal gas law, the pressure must have units of atmospheres and the temperature must have units of Kelvin.
Convert the given pressure into atmospheres:


Now, substitute in all the given values in order to find the number of moles of nitrogen gas.

Next, use the stoichiometric ratio given by the chemical equation to find the number of moles of lithium needed to react completely with the nitrogen gas.

Finally, convert the number of moles of lithium to number of grams of lithium.

Start by finding the number of moles of nitrogen gas by using the ideal gas law:
Rearrange the equation to solve for the variable 
In order to use the ideal gas law, the pressure must have units of atmospheres and the temperature must have units of Kelvin.
Convert the given pressure into atmospheres:
Now, substitute in all the given values in order to find the number of moles of nitrogen gas.
Next, use the stoichiometric ratio given by the chemical equation to find the number of moles of lithium needed to react completely with the nitrogen gas.
Finally, convert the number of moles of lithium to number of grams of lithium.
Compare your answer with the correct one above
What is the pressure exerted by  in a
in a  container at
container at  ?
?
What is the pressure exerted by 


Compare your answer with the correct one above
Avogadro's Laws relate which of the following variables?
Avogadro's Laws relate which of the following variables?
Avogadro's law states that volume is proportional to moles of gas. Temperature and pressure are fixed. Boyle law states that volume of gas is inversely proportional to pressure. Charles' law states that volume of gas is proportional to temperature.
Avogadro's law states that volume is proportional to moles of gas. Temperature and pressure are fixed. Boyle law states that volume of gas is inversely proportional to pressure. Charles' law states that volume of gas is proportional to temperature.
Compare your answer with the correct one above
Ideal gas law calculation

The given equation shows the synthesis of methanol. Find the volume in liters of  required to synthesize
required to synthesize  of methanol at
of methanol at  and a pressure of
and a pressure of  .
.
Ideal gas law calculation
The given equation shows the synthesis of methanol. Find the volume in liters of 



Use the ideal gas equation

Rearrange to solve for the volume



Use the ideal gas equation
Rearrange to solve for the volume
Compare your answer with the correct one above
What is the volume occupied by  of helium gas at a pressure of
of helium gas at a pressure of  and a temperature of
and a temperature of  degrees celsius?
degrees celsius?
What is the volume occupied by 


Recall the Ideal Gas Law:

Since the question is asking for volume, rearrange the equation to the following:

Also recall that for the Ideal Gas Law equation to work, the temperature must be given in degrees Kelvin, and we will also need the number of moles of gas.
Start by converting the mass of gas into moles:

Don't round the values just yet.
Next, convert the temperature into degrees Kelvin.

Now, plug in all the given values into the equation to solve for volume:

The answer should have  significant figures.
significant figures.
The volume is  .
.
Recall the Ideal Gas Law:
Since the question is asking for volume, rearrange the equation to the following:
Also recall that for the Ideal Gas Law equation to work, the temperature must be given in degrees Kelvin, and we will also need the number of moles of gas.
Start by converting the mass of gas into moles:
Don't round the values just yet.
Next, convert the temperature into degrees Kelvin.
Now, plug in all the given values into the equation to solve for volume:
The answer should have 
The volume is 
Compare your answer with the correct one above
A cylinder contains  of oxygen gas at a pressure of
of oxygen gas at a pressure of  and a temperature of
and a temperature of  . How many grams of gas are in the cylinder?
. How many grams of gas are in the cylinder?
A cylinder contains 


Recall the Ideal Gas Law:

Because we want the amount of gas in the cylinder, we must find the number of moles of oxygen present first. Rearrange the Ideal Gas Law to solve for the number of moles of oxygen.

Plug in the given information to solve for the number of moles.

Now, convert the number of moles to the number of grams by using the molar mass of oxygen gas.

Make sure that your answer has  significant figures.
significant figures.
Recall the Ideal Gas Law:
Because we want the amount of gas in the cylinder, we must find the number of moles of oxygen present first. Rearrange the Ideal Gas Law to solve for the number of moles of oxygen.
Plug in the given information to solve for the number of moles.
Now, convert the number of moles to the number of grams by using the molar mass of oxygen gas.
Make sure that your answer has 
Compare your answer with the correct one above
A gas fills a  container, where heat is added raising its temperature from
container, where heat is added raising its temperature from  to
to  and it increases its pressure from
and it increases its pressure from  to
to  . What is the gas’ new volume?
. What is the gas’ new volume?
A gas fills a 




Use the combined gas law:






Solve for  since that is the new volume for which we need to solve:
since that is the new volume for which we need to solve:



Use the combined gas law:
Solve for 
Compare your answer with the correct one above
An ideal gas with a pressure of  is expanded to
is expanded to  times its original volume, what is the new pressure?
times its original volume, what is the new pressure?
An ideal gas with a pressure of 

Use Boyle’s law:

You want to solve for the new pressure so isolate  :
:


It expands to  its original volume, so
its original volume, so 
Plug in everything we know:

Cancel  from both the numerator and denominator
from both the numerator and denominator


Use Boyle’s law:
You want to solve for the new pressure so isolate 
It expands to 
Plug in everything we know:
Cancel 
Compare your answer with the correct one above
An ideal gas with a pressure of  is compressed to
is compressed to  of its original volume, what is the new pressure?
of its original volume, what is the new pressure?
An ideal gas with a pressure of 

An ideal gas with a pressure of  is is compressed to
is is compressed to  of its original volume, what is the new pressure?
of its original volume, what is the new pressure?
Use Boyle’s law:

You want to solve for the new pressure so isolate  :
:


It compresses to  its original volume so,
its original volume so, 
Plug in everything we know:

Cancel  from the numerator and denominator
from the numerator and denominator


An ideal gas with a pressure of 

Use Boyle’s law:
You want to solve for the new pressure so isolate 
It compresses to 
Plug in everything we know:
Cancel 
Compare your answer with the correct one above
One flask is at STP and another is at  . What is the pressure at
. What is the pressure at 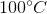 ?
?
One flask is at STP and another is at 
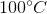
The pressure of the flask at  is
is  .
.

Because the volume between the flasks and the moles in each flask are constant, we can cancel out  and
and  .
.

At STP, conditions are  and
and  .
.
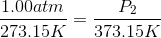

The pressure of the flask at 

Because the volume between the flasks and the moles in each flask are constant, we can cancel out 

At STP, conditions are 

Compare your answer with the correct one above
A 3.00 L container at 273 K is filled with 1.00 mol Cl2(g), which behaves non-ideally.
Using the van der Waals equation, calculate pressure exerted by the gas.


A 3.00 L container at 273 K is filled with 1.00 mol Cl2(g), which behaves non-ideally.
Using the van der Waals equation, calculate pressure exerted by the gas.
Recall the van der Waals equation for non-ideal gases
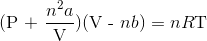
Rewrite the equation using the known values and solve for P
![[\textrm{P + }\frac{(1\textrm{ mol})^{2}(6.49\frac{\textrm{L}^{2}\textrm{ atm}}{\textrm{mol}^{2}})}{(3.00\textrm{ L})^2}][\textrm{3.00 L - (1 mol)}(0.0562\frac{\textrm{L}}{\textrm{mol}})]=(1\textrm{ mol})^{2}(0.0821\frac{\textrm{atm}}{\textrm{mol K}})(273K)](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/996927/gif.latex)


Recall the van der Waals equation for non-ideal gases
Rewrite the equation using the known values and solve for P
Compare your answer with the correct one above
A  mixture of helium, nitrogen, and neon has a total pressure of
mixture of helium, nitrogen, and neon has a total pressure of  at a temperature of
at a temperature of  . If the partial pressure of helium is
. If the partial pressure of helium is  and the partial pressure of nitrogen is
and the partial pressure of nitrogen is  , what mass of neon is present in the mixture?
, what mass of neon is present in the mixture?
A 




Since we have the total pressure and the pressures of helium and nitrogen, we can find the pressure of neon in this container.



Next, convert this pressure into atmospheres.

Now, recall the Ideal Gas Law:

Rearrange the equation to solve for moles of gas:

Using the pressure of neon, find the moles of neon that is present in the container.

Finally, convert the moles of neon into grams of neon using the molar mass.

Make sure your answer has  significant digits.
significant digits.
Since we have the total pressure and the pressures of helium and nitrogen, we can find the pressure of neon in this container.
Next, convert this pressure into atmospheres.
Now, recall the Ideal Gas Law:
Rearrange the equation to solve for moles of gas:
Using the pressure of neon, find the moles of neon that is present in the container.
Finally, convert the moles of neon into grams of neon using the molar mass.
Make sure your answer has 
Compare your answer with the correct one above
Suppose that hydrochloric acid and aluminum are reacted to produce hydrogen gas. This hydrogen gas is collected by displacement of water at  and a total pressure of
and a total pressure of  . If the volume of the gas collected is
. If the volume of the gas collected is  , then how many moles of hydrogen were collected?
, then how many moles of hydrogen were collected?
Note: The vapor pressure of water at  is
is  .
.
Suppose that hydrochloric acid and aluminum are reacted to produce hydrogen gas. This hydrogen gas is collected by displacement of water at 


Note: The vapor pressure of water at 

For this question, we're told that a chemical reaction is producing hydrogen gas. In doing so, the gas is displacing a certain amount of water vapor. We're asked to determine the number of moles of gas produced in this process.
The amount of water displaced by the production of hydrogen gas will be the amount (volume) of hydrogen gas produced. To measure its pressure, we take the reading of the total pressure and then subtract from it the pressure of water vapor (since it contributes to the total pressure). Using this information, we can use the ideal gas equation to determine the number of moles of hydrogen gas.




Next, we can use the ideal gas equation to solve for our answer.




For this question, we're told that a chemical reaction is producing hydrogen gas. In doing so, the gas is displacing a certain amount of water vapor. We're asked to determine the number of moles of gas produced in this process.
The amount of water displaced by the production of hydrogen gas will be the amount (volume) of hydrogen gas produced. To measure its pressure, we take the reading of the total pressure and then subtract from it the pressure of water vapor (since it contributes to the total pressure). Using this information, we can use the ideal gas equation to determine the number of moles of hydrogen gas.
Next, we can use the ideal gas equation to solve for our answer.
Compare your answer with the correct one above

