Reactions - College Chemistry
Card 0 of 20
Determine the oxidation number of each element in the compound 
Determine the oxidation number of each element in the compound
The oxidation number of each element in the compound  is:
is:


The oxidation number of chlorine is  . There are 3 chlorine atoms present in the compound, so
. There are 3 chlorine atoms present in the compound, so 
Because this is a neutral atom, the overall charge is 0. Therefore we can set 
Therefore, 
We can then solve for the oxidation number for 

The oxidation number of each element in the compound 
The oxidation number of chlorine is 
Because this is a neutral atom, the overall charge is 0. Therefore we can set
Therefore,
We can then solve for the oxidation number for
Compare your answer with the correct one above
Given the following equation, identify the reducing agent.

Given the following equation, identify the reducing agent.
Recall that a reducing agent is being oxidized and is losing electrons. Thus, when looking at the equation, look for which oxidation state is becoming more positive.
Start by assigning oxidation states to the elements.
For the reactants:
 has an oxidation state of
has an oxidation state of  .
.
 has an oxidation state of
has an oxidation state of  .
.
 has an oxidation state of
has an oxidation state of 
 has an oxidation state of
has an oxidation state of  .
.
Now, assign the oxidation state of the products.
 has an oxidation state of
has an oxidation state of  .
.
 has an oxidation state of
has an oxidation state of  .
.
 has an oxidation state of
has an oxidation state of  .
.
 has an oxidation state of
has an oxidation state of  .
.
Only  undergoes a loss of electrons. It must be the reducing agent.
undergoes a loss of electrons. It must be the reducing agent.
Recall that a reducing agent is being oxidized and is losing electrons. Thus, when looking at the equation, look for which oxidation state is becoming more positive.
Start by assigning oxidation states to the elements.
For the reactants:







Now, assign the oxidation state of the products.








Only 
Compare your answer with the correct one above
Which compound below has a nitrogen atom at a  oxidation state?
oxidation state?
Which compound below has a nitrogen atom at a 
Let's start by going through each answer case-by-case applying the elementary rules of oxidation states we are given. The most applicable of these rules in this problem are the oxidation state of hydrogen in a compound is generally equal to  , and the oxidation state of oxygen in a compound is generally equal to
, and the oxidation state of oxygen in a compound is generally equal to  .
.
Let's start by looking at  . We know this compound as a whole is neutrally charged (equal to
. We know this compound as a whole is neutrally charged (equal to  overall charge), and applying our knowledge of the general oxidation state of hydrogen in a compound and knowing there are
overall charge), and applying our knowledge of the general oxidation state of hydrogen in a compound and knowing there are  hydrogens we know that the
hydrogens we know that the  hydrogens have a total charge of
hydrogens have a total charge of  together, and nitrogen has an unknown oxidation state
together, and nitrogen has an unknown oxidation state  , so for our equation we have:
, so for our equation we have:

solving for  gives
gives  , giving us the answer we need. Therefore the nitrogen atom in
, giving us the answer we need. Therefore the nitrogen atom in  has an oxidation state of
has an oxidation state of  , which is the correct answer.
, which is the correct answer.
Let's still look at the other cases, however:

As this compound is uncharged we know it's net charge is equal to 
Using our knowledge of oxidation states of hydrogen and oxygen and counting the number of hydrogens and oxygen in this compound, we can determine that the total charge of all the hydrogens together is equal to  , and that the total charge of the one oxygen is
, and that the total charge of the one oxygen is  .
.
Just as we did above we can create an equation and solve for our unknown.

therefore  , so the oxidation state of nitrogen in
, so the oxidation state of nitrogen in  is
is  . This means this isn't the right compound.
. This means this isn't the right compound.
Next let's look at  :
:
We can quickly apply our knowledge of oxidation states of oxygen knowing that the oxidation state of oxygen in compounds is generally  . Since there are
. Since there are  oxygens we must multiply
oxygens we must multiply  to find out the total charge contributed by all the oxygens, which is
to find out the total charge contributed by all the oxygens, which is  .
.
So our equation for this becomes:

therefore  and the oxidation state of nitrogen is
and the oxidation state of nitrogen is  , which isn't the answer we are looking for.
, which isn't the answer we are looking for.
Finally the last compound is  :
:
With this compound we can apply the rule we know about hydrogen's oxidation state in a compound being equal to  . Since there are
. Since there are  hydrogens, we must multiply
hydrogens, we must multiply  which equals
which equals  .
.
Since there are two nitrogens, our equation is:

and  . Therefore the oxidation state of nitrogen is equal to
. Therefore the oxidation state of nitrogen is equal to  .
.
Let's start by going through each answer case-by-case applying the elementary rules of oxidation states we are given. The most applicable of these rules in this problem are the oxidation state of hydrogen in a compound is generally equal to 

Let's start by looking at 





solving for 



Let's still look at the other cases, however:
As this compound is uncharged we know it's net charge is equal to
Using our knowledge of oxidation states of hydrogen and oxygen and counting the number of hydrogens and oxygen in this compound, we can determine that the total charge of all the hydrogens together is equal to 

Just as we did above we can create an equation and solve for our unknown.
therefore 


Next let's look at 
We can quickly apply our knowledge of oxidation states of oxygen knowing that the oxidation state of oxygen in compounds is generally 



So our equation for this becomes:
therefore 

Finally the last compound is 
With this compound we can apply the rule we know about hydrogen's oxidation state in a compound being equal to 



Since there are two nitrogens, our equation is:
and 

Compare your answer with the correct one above
In the given reaction, which element(s) is/are oxidized?

In the given reaction, which element(s) is/are oxidized?
Let's start by looking at the equation and assigning oxidation numbers based off of the general rules we are given:

Let's start by looking at the reactants, namely  . Knowing that an atom in its elemental form has an oxidation number of
. Knowing that an atom in its elemental form has an oxidation number of  ,
,  has an oxidation number equal to
has an oxidation number equal to  .
.
Next let's look at  . This is a neutral compound so know the oxidation number of the whole compound must equal
. This is a neutral compound so know the oxidation number of the whole compound must equal  . Going off of our general rules, the oxidation number of fluorine is equal
. Going off of our general rules, the oxidation number of fluorine is equal  . Since we have 3 fluorines we multiply the oxidation number by
. Since we have 3 fluorines we multiply the oxidation number by  to get the cumulative oxidation number of all the fluorines together to get
to get the cumulative oxidation number of all the fluorines together to get  . So now we must solve for the oxidation of Cl, which is unknown. This is done through a simple algebraic equation:
. So now we must solve for the oxidation of Cl, which is unknown. This is done through a simple algebraic equation: 
so  . Therefore the oxidation number of Chlorine is
. Therefore the oxidation number of Chlorine is  .
.
Now we have the oxidation numbers of all the elements present in this equation that are on the reactant side. I recommend writing down their oxidation numbers next to each other, so the elements that are oxidized and reduced can quickly be determined.
Oxidation numbers of elements in reactants:



Now let's look at the product side of the equation and determine the oxidation numbers of the elements there. First let's look at  . Knowing the general rules of oxidation numbers we know that fluorine has an oxidation number of
. Knowing the general rules of oxidation numbers we know that fluorine has an oxidation number of  . Since there are
. Since there are  fluorines we multiply
fluorines we multiply  which equals
which equals  . We can now solve for the oxidation number of Uranium.
. We can now solve for the oxidation number of Uranium.

 , therefore
, therefore  has an oxidation number of
has an oxidation number of  .
.
Finally, let's look  . We can once again use the general rule of oxidation numbers that fluorine has a
. We can once again use the general rule of oxidation numbers that fluorine has a  oxidation number to simplify this. Therefore
oxidation number to simplify this. Therefore 
 , therefore Cl has an oxidation number of
, therefore Cl has an oxidation number of  in the product.
in the product.
Let's now write down the oxidation numbers of all the elements in the products.
Oxidation numbers of elements in products:



Now let's compare the oxidation numbers of the elements in the reactants with those in the products.  goes from a
goes from a  oxidation number to a
oxidation number to a  , therefore it is the element that's oxidized (oxidation represents a loss in electrons).
, therefore it is the element that's oxidized (oxidation represents a loss in electrons).  goes from
goes from  to
to  , therefore it is reduced (reduction means gaining electrons). Finally
, therefore it is reduced (reduction means gaining electrons). Finally  's oxidation number is
's oxidation number is  in both the products and reactants therefore it is neither oxidized nor reduced. This means that
in both the products and reactants therefore it is neither oxidized nor reduced. This means that  is the only element that's oxidized.
is the only element that's oxidized.
Let's start by looking at the equation and assigning oxidation numbers based off of the general rules we are given:
Let's start by looking at the reactants, namely 



Next let's look at 




so 

Now we have the oxidation numbers of all the elements present in this equation that are on the reactant side. I recommend writing down their oxidation numbers next to each other, so the elements that are oxidized and reduced can quickly be determined.
Oxidation numbers of elements in reactants:
Now let's look at the product side of the equation and determine the oxidation numbers of the elements there. First let's look at 







Finally, let's look 



Let's now write down the oxidation numbers of all the elements in the products.
Oxidation numbers of elements in products:
Now let's compare the oxidation numbers of the elements in the reactants with those in the products. 








Compare your answer with the correct one above
In which of the following compounds is the oxidation state of phosphorus the least?
In which of the following compounds is the oxidation state of phosphorus the least?
Let's start by examining each of the compounds we are given case-by-case and applying the general rules of oxidation numbers we know.
Let's start with  :
:
We know that based off of the general rules of oxidation states, Oxygen generally has an oxidation number of  , since we have
, since we have  oxygens we must multiply
oxygens we must multiply  to get the total cumulative charge of all the oxygens. This equals
to get the total cumulative charge of all the oxygens. This equals  .
.
Now we can solve for the oxidation state of phosphorus:
Since we have  Phosphoruses we multiply our unknown
Phosphoruses we multiply our unknown  by
by  .
.
 .
.
This is equal to  because the compound has an overall
because the compound has an overall  net charge.
net charge.
Solving for  gives you
gives you  therefore the oxidation state of Phosphorus in this compound is equal to
therefore the oxidation state of Phosphorus in this compound is equal to  .
.
Next let's looking at  :
:
Knowing that an atom in its elemental form has an oxidation state of  . This compound has an oxidation number of
. This compound has an oxidation number of  .
.
Now let's look at  :
:
Knowing that the oxidation number of hydrogen is  and that there are
and that there are  hydrogens, the total oxidation of all the hydrogens together is
hydrogens, the total oxidation of all the hydrogens together is  , therefore our equation is:
, therefore our equation is:

so  , therefore the oxidation state of Phosphorus in this compound is
, therefore the oxidation state of Phosphorus in this compound is  .
.
Now let's consider  :
:
Using our elementary rules for the oxidation numbers, we know oxygen has a  oxidation number. We can also determine the oxidation number of chlorine, using the rule stating that the oxidation number of halogens is usually
oxidation number. We can also determine the oxidation number of chlorine, using the rule stating that the oxidation number of halogens is usually  . Since there are three chlorines we must multiply
. Since there are three chlorines we must multiply  , which gives us
, which gives us  .
.
Therefore our equation is:

therefore  , so the oxidation number of phosphorus in this case equals
, so the oxidation number of phosphorus in this case equals  .
.
Finally let's look at  :
:
Using the rules stated above for  , we obtain the equation:
, we obtain the equation:

Therefore  meaning the oxidation number of
meaning the oxidation number of  .
.
This means that the compound with the lowest oxidation state is  , therefore it is the right answer.
, therefore it is the right answer.
Let's start by examining each of the compounds we are given case-by-case and applying the general rules of oxidation numbers we know.
Let's start with 
We know that based off of the general rules of oxidation states, Oxygen generally has an oxidation number of 



Now we can solve for the oxidation state of phosphorus:
Since we have 



This is equal to 

Solving for 


Next let's looking at 
Knowing that an atom in its elemental form has an oxidation state of 

Now let's look at 
Knowing that the oxidation number of hydrogen is 


so 

Now let's consider 
Using our elementary rules for the oxidation numbers, we know oxygen has a 



Therefore our equation is:
therefore 

Finally let's look at 
Using the rules stated above for 
Therefore 

This means that the compound with the lowest oxidation state is 
Compare your answer with the correct one above
What is the oxidation state of gold in  ?
?
What is the oxidation state of gold in 
Let's first consider the overall compound  . Since it is negatively charged overall it is equal to
. Since it is negatively charged overall it is equal to  . Applying our elementary rules of oxidation states we know that halogens generally have an oxidation state of
. Applying our elementary rules of oxidation states we know that halogens generally have an oxidation state of  , so this means chlorine has an oxidation state of
, so this means chlorine has an oxidation state of  . Since there are
. Since there are  chlorines we must multiply
chlorines we must multiply  to get the charge of all the chlorines together. This is equal to
to get the charge of all the chlorines together. This is equal to  .
.
We can now solve for oxidation state of gold through creating an equation as shown below:

Simplifying this we get that  , therefore the oxidation state of gold is equal to
, therefore the oxidation state of gold is equal to 
Let's first consider the overall compound 






We can now solve for oxidation state of gold through creating an equation as shown below:
Simplifying this we get that 
Compare your answer with the correct one above
What species is reduced in the following chemical reaction?

What species is reduced in the following chemical reaction?
Reduction is defined as the gain of electrons so we want to find the element that gains electrons/becomes negatively charged.
Once again our equation is 
Let's start on the reactant side with  :
:
Following our general rules of oxidation states, we know that an atom in its elemental form has an oxidation state equal to zero, therefore  has an oxidation state equal to
has an oxidation state equal to  .
.
Next, consider  : Based off of our general oxidation state rules we known the oxidation number of oxygen is
: Based off of our general oxidation state rules we known the oxidation number of oxygen is  , since we have two oxygens we must multiply
, since we have two oxygens we must multiply  . This means that the overall combined charge of the oxygens in this compound is equal to
. This means that the overall combined charge of the oxygens in this compound is equal to  .
.
We can now solve for the oxidation state of  using this equation, which set equal to zero because the net charge of the compound is
using this equation, which set equal to zero because the net charge of the compound is  .
.

 , therefore
, therefore 
Now let's consider  :
:
Applying the rule for the oxidation number of oxygen being  and the rule of hydrogen being equal to +1, we obtain the equation:
and the rule of hydrogen being equal to +1, we obtain the equation:

where  is the oxidation number of sulfur.
is the oxidation number of sulfur.
This simplifies so that  , therefore the oxidation number of
, therefore the oxidation number of  .
.
Moving on to the products, let's consider  :
:
We know that the oxidation state of Oxygen is  based off the rules mentioned above. We can also determine that the oxidation number of
based off the rules mentioned above. We can also determine that the oxidation number of  is equal to
is equal to  because lead is most stable when it loses two electrons.
because lead is most stable when it loses two electrons.
Now we must solve for sulfur's oxidation state:

therefore  , therefore the oxidation state of sulfur
, therefore the oxidation state of sulfur  .
.
As for  , we can just use the general oxidation rules mentioned above to determine
, we can just use the general oxidation rules mentioned above to determine 

Looking at the changes of oxidation numbers between elements in the products and reactants we see that there is no change in the oxidation state of sulfur, hydrogen, and oxygen between the products and reactants, but lead goes from  to
to  , so therefore it is reduced.
, so therefore it is reduced.
Reduction is defined as the gain of electrons so we want to find the element that gains electrons/becomes negatively charged.
Once again our equation is
Let's start on the reactant side with 
Following our general rules of oxidation states, we know that an atom in its elemental form has an oxidation state equal to zero, therefore 

Next, consider 



We can now solve for the oxidation state of 


Now let's consider 
Applying the rule for the oxidation number of oxygen being 
where 
This simplifies so that 

Moving on to the products, let's consider 
We know that the oxidation state of Oxygen is 


Now we must solve for sulfur's oxidation state:
therefore 

As for 

Looking at the changes of oxidation numbers between elements in the products and reactants we see that there is no change in the oxidation state of sulfur, hydrogen, and oxygen between the products and reactants, but lead goes from 

Compare your answer with the correct one above
What is the oxidation state of copper in  ?
?
What is the oxidation state of copper in 
In order to determine the oxidation state of copper in this compound we first must note the fact that compound has an overall charge of  .
.
We can also apply the general rules of oxidation numbers we know to determine the oxidation numbers of hydrogen and oxygen. Since hydrogen is a group  element, it has an oxidation number of
element, it has an oxidation number of  . Since there are two hydrogens we must multiple
. Since there are two hydrogens we must multiple  which equals
which equals  to get the total charge of both hydrogens.
to get the total charge of both hydrogens.
As for oxygen, based off of our general rules of oxidation numbers, we know that oxygen normally has an oxidation number of  , so knowing the oxidation numbers of oxygen and hydrogen, and net the charge of the compound, we can setup an equation to solve for the oxidation number of copper as shown below:
, so knowing the oxidation numbers of oxygen and hydrogen, and net the charge of the compound, we can setup an equation to solve for the oxidation number of copper as shown below:

therefore  , therefore copper has an oxidation number equal to
, therefore copper has an oxidation number equal to  .
.
In order to determine the oxidation state of copper in this compound we first must note the fact that compound has an overall charge of 
We can also apply the general rules of oxidation numbers we know to determine the oxidation numbers of hydrogen and oxygen. Since hydrogen is a group 



As for oxygen, based off of our general rules of oxidation numbers, we know that oxygen normally has an oxidation number of 
therefore 

Compare your answer with the correct one above
What is the oxidation number of sulfur in  ?
?
What is the oxidation number of sulfur in 
In order to determine the oxidation number we must apply the general rules of oxidation numbers we know. One of these rules states the oxidation number of fluorine is always  . Since there are
. Since there are  fluorines that means the fluorines together contribute a
fluorines that means the fluorines together contribute a  charge to the compound. Since the overall charge of the compound is
charge to the compound. Since the overall charge of the compound is  , we can create an equation with an unknown (charge of sulfur) and solve for it to find the oxidation number of sulfur.
, we can create an equation with an unknown (charge of sulfur) and solve for it to find the oxidation number of sulfur.
This equation is  The reason why it is
The reason why it is  instead of just
instead of just  is because there are
is because there are  sulfurs.
sulfurs.

therefore the oxidation number of sulfur is  .
.
In order to determine the oxidation number we must apply the general rules of oxidation numbers we know. One of these rules states the oxidation number of fluorine is always 



This equation is 



therefore the oxidation number of sulfur is 
Compare your answer with the correct one above
When balanced, what is the value of  in the following chemical equation?
in the following chemical equation?

When balanced, what is the value of 
Recall that a balanced chemical equation has the same number of each element on one side.

Start by counting the number of each element on each side.
There are the following numbers of moles of each reactant:




There are the following numbers of moles of each product:




Add coefficients in front of the molecular compounds in the equation until there are the same numbers of sulfur, oxygen, lithium, and selenium on each side.
The balanced chemical equation is the following:

Both products and reactants now have the following number of moles:




Since the coefficient in front of  is
is  ,
,  must equal to
must equal to  .
.
Recall that a balanced chemical equation has the same number of each element on one side.
Start by counting the number of each element on each side.
There are the following numbers of moles of each reactant:
There are the following numbers of moles of each product:
Add coefficients in front of the molecular compounds in the equation until there are the same numbers of sulfur, oxygen, lithium, and selenium on each side.
The balanced chemical equation is the following:
Both products and reactants now have the following number of moles:
Since the coefficient in front of 



Compare your answer with the correct one above
Balance the following chemical equation:

Balance the following chemical equation:
A balanced chemical equation will have the same number of each atom on both sides of the reaction.

Start by balancing the number of nitrate.
Since we have  nitrate on the left, we must also have
nitrate on the left, we must also have  nitrate on the right.
nitrate on the right.

This equation now gives  potassium on the left, and
potassium on the left, and  potassium on the right. Balance the potassium.
potassium on the right. Balance the potassium.

This equation is balanced because there are equal numbers of each atom on both sides.
A balanced chemical equation will have the same number of each atom on both sides of the reaction.
Start by balancing the number of nitrate.
Since we have 

This equation now gives 

This equation is balanced because there are equal numbers of each atom on both sides.
Compare your answer with the correct one above
Balance the following redox reaction in acidic conditions:

Balance the following redox reaction in acidic conditions:
Start by writing the half reactions for the equation


All atoms except for O and H are already balanced, so we can go straight to balancing O and H atoms. First balance O atoms by adding H2O
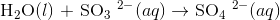

Now balance H atoms with H+


Balance each half reaction's electrical charge with electrons


Now combine both half reactions to get the overall reaction. Be sure to cancel out electrons by multiplying the oxidation reaction by 5 and the reduction reaction by 2. This will give 10 e- on each side of the overall reaction so that they cancel out.


Overall Reaction:


Notice that we have H2O and H+ on both sides of the equation, so we need to simplify. Subtract the 5 H2O on the reactant side from the 8 H2O on the product side which leaves 3 H2O on the reactant side. Then subtract the 10 H+ on the product side from the 16 H+ on the reactant side which leave 6 H+ on the reactant side. Now we have the simplified overall reaction

Start by writing the half reactions for the equation
All atoms except for O and H are already balanced, so we can go straight to balancing O and H atoms. First balance O atoms by adding H2O
Now balance H atoms with H+
Balance each half reaction's electrical charge with electrons
Now combine both half reactions to get the overall reaction. Be sure to cancel out electrons by multiplying the oxidation reaction by 5 and the reduction reaction by 2. This will give 10 e- on each side of the overall reaction so that they cancel out.
Overall Reaction:
Notice that we have H2O and H+ on both sides of the equation, so we need to simplify. Subtract the 5 H2O on the reactant side from the 8 H2O on the product side which leaves 3 H2O on the reactant side. Then subtract the 10 H+ on the product side from the 16 H+ on the reactant side which leave 6 H+ on the reactant side. Now we have the simplified overall reaction
Compare your answer with the correct one above
Determine the pH of an aqueous solution of 0.01 M acetic acid,  . The pKa of acetic acid is 4.75.
. The pKa of acetic acid is 4.75.
Determine the pH of an aqueous solution of 0.01 M acetic acid, 
Since acetic acid is a weak acid, it has a Ka that is rather small, we have to do a RICE table to determine the equilibrium amount of hydronium, H3O+ to then determine the pH.
R 
I 0.1 M - 0 0
C -x +x +x
E 0.1 -x x x
So first we need to change our pKa to a Ka
where  therefore
therefore

 =
= ![\frac{[CH_{}3COO^{}-][H_{}3O^{}+]}{[CH_{}3COOH]}](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/1006241/gif.latex) =
= 
If we assume that x is very small compared to 0.1...

Where 
(note: when solving using the quadratic we come up with the same answer)
So if ![x= 0.00133 = [H_{}3O^{}+]](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/1006245/gif.latex)
![pH = -log [H_{}3O^{}+] =-log(0.0013) = 2.87](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/1006246/gif.latex)
Since acetic acid is a weak acid, it has a Ka that is rather small, we have to do a RICE table to determine the equilibrium amount of hydronium, H3O+ to then determine the pH.
R
I 0.1 M - 0 0
C -x +x +x
E 0.1 -x x x
So first we need to change our pKa to a Ka
where 

![\frac{[CH_{}3COO^{}-][H_{}3O^{}+]}{[CH_{}3COOH]}](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/1006241/gif.latex)
If we assume that x is very small compared to 0.1...
Where
(note: when solving using the quadratic we come up with the same answer)
So if
Compare your answer with the correct one above
Which combination(s) would create a buffer solution?
I. Weak acid
II. Weak acid's conjugate base
III. Strong acid
IV. Strong base
V. Weak base
VI. Weak base's conjugate acid
Which combination(s) would create a buffer solution?
I. Weak acid
II. Weak acid's conjugate base
III. Strong acid
IV. Strong base
V. Weak base
VI. Weak base's conjugate acid
A buffer solution is formed from the equilibrium of a weak acid and its conjugate base, or from a weak base and its conjugate acid. It's ability to "buffer" the pH or keep it from changing in large amounts in from the switching between these two forms weak and its conjugate.
A buffer solution is formed from the equilibrium of a weak acid and its conjugate base, or from a weak base and its conjugate acid. It's ability to "buffer" the pH or keep it from changing in large amounts in from the switching between these two forms weak and its conjugate.
Compare your answer with the correct one above
You are in chemistry lab performing a titration. You were given 15 mL of an aqueous solution with an unknown concentration of acetic acid,  to solve through titration with concentrated sodium hydroxide,
to solve through titration with concentrated sodium hydroxide,  . You know that the pKa of acetic acid is 4.75 and that your titrant is 0.1 M sodium hydroxide,
. You know that the pKa of acetic acid is 4.75 and that your titrant is 0.1 M sodium hydroxide,  .
.
The endpoint was determined at 10 mL of sodium hydroxide,  . What is the pH after 5 mL of
. What is the pH after 5 mL of  was added?
was added?
You are in chemistry lab performing a titration. You were given 15 mL of an aqueous solution with an unknown concentration of acetic acid, 


The endpoint was determined at 10 mL of sodium hydroxide, 

At the half end point, the  . This can be determined by the Henderson-Hasselbalch equation if it is not clear.
. This can be determined by the Henderson-Hasselbalch equation if it is not clear.
Since the endpoint of the titration is that there are 10 mL of 0.1 M NaOH added, that means that there are 0.001 moles of acetic acid.
When 5 mL of NaOH is added, there are 0.0005 moles of acetic acid and 0.0005 moles of acetate formed.


Therefore pH= pKa
At the half end point, the 
Since the endpoint of the titration is that there are 10 mL of 0.1 M NaOH added, that means that there are 0.001 moles of acetic acid.
When 5 mL of NaOH is added, there are 0.0005 moles of acetic acid and 0.0005 moles of acetate formed.
Therefore pH= pKa
Compare your answer with the correct one above
Determine which of these solution combinations form a buffer.
Determine which of these solution combinations form a buffer.
First to go through why the other ones are wrong:
Strong base + strong acid neutralizes and does not form a buffer solution
Strong base + weak base does not form a buffer - would need an acid
Strong base + weak acid = all weak acid converted to conjugate base
The correct answer is:
Strong base + weak acid = half converted to conjugate base with half leftover as weak acid, with all the components for a buffer
First to go through why the other ones are wrong:
Strong base + strong acid neutralizes and does not form a buffer solution
Strong base + weak base does not form a buffer - would need an acid
Strong base + weak acid = all weak acid converted to conjugate base
The correct answer is:
Strong base + weak acid = half converted to conjugate base with half leftover as weak acid, with all the components for a buffer
Compare your answer with the correct one above
Determine which combination of solutions would create a buffer solution.
Determine which combination of solutions would create a buffer solution.
For all the other options there is no ammonium leftover with which to serve as the weak acid in the buffer system, the ammonium is all used up and converted to ammonia. However in the correct answer choice, there is enough ammonium leftover after the reaction with the sodium hydroxide.
For all the other options there is no ammonium leftover with which to serve as the weak acid in the buffer system, the ammonium is all used up and converted to ammonia. However in the correct answer choice, there is enough ammonium leftover after the reaction with the sodium hydroxide.
Compare your answer with the correct one above
Determine which solution(s) will yield a buffer solution.
I. 10 mL of 0.5 M HCl + 20 mL of 0.5 M acetate
II. 10 mL of 0.5 M HCl + 10 mL of 0.5 M acetate
III. 10 mL of 0.5 M HCl + 10 mL of 1.0 M acetate
IV. 10 mL of 0.5 M HCl + 10 mL of 1.5 M acetate
Determine which solution(s) will yield a buffer solution.
I. 10 mL of 0.5 M HCl + 20 mL of 0.5 M acetate
II. 10 mL of 0.5 M HCl + 10 mL of 0.5 M acetate
III. 10 mL of 0.5 M HCl + 10 mL of 1.0 M acetate
IV. 10 mL of 0.5 M HCl + 10 mL of 1.5 M acetate
These answers are correct because the two components needed to create a buffer solution are a weak acid and its conjugate base, or a weak base and its conjugate acid. In these cases, the first reaction to occur upon addition of the strong acid is the formation of the conjugate acid, acetic acid.

If the amount of initial  is greater than HCl, then we will have some
is greater than HCl, then we will have some  left over to act as a buffer with the created conjugate acid. This can be through a greater volume, or through a higher concentration as shown in the correct answers.
left over to act as a buffer with the created conjugate acid. This can be through a greater volume, or through a higher concentration as shown in the correct answers.
These answers are correct because the two components needed to create a buffer solution are a weak acid and its conjugate base, or a weak base and its conjugate acid. In these cases, the first reaction to occur upon addition of the strong acid is the formation of the conjugate acid, acetic acid.
If the amount of initial 

Compare your answer with the correct one above
Which of the following acid and base pairs are capable of acting as a buffer?
Which of the following acid and base pairs are capable of acting as a buffer?
In this question, we're presented with a variety of acid/base pairs and we're asked to identify which one could act as a buffer.
Remember that a buffer is a pair of acid and its conjugate base that acts to resist substantial changes in pH. In order for a buffer to work, the acid base pair needs to exist in equilibrium. This way, when the pH of the solution changes, the equilibrium of the acid/base reaction will shift, such that the pH will not change drastically.
To have an acid/base pair in equilibrium, we'll need to look for a pair that contains a weak acid. Acids like  and
and  are so strong that they will dissociate completely. Of the answer choices shown, only the carbonic acid/bicarbonate system (
are so strong that they will dissociate completely. Of the answer choices shown, only the carbonic acid/bicarbonate system ( and
and  ) exists in equilibrium. Thus, this is the correct answer.
) exists in equilibrium. Thus, this is the correct answer.
In this question, we're presented with a variety of acid/base pairs and we're asked to identify which one could act as a buffer.
Remember that a buffer is a pair of acid and its conjugate base that acts to resist substantial changes in pH. In order for a buffer to work, the acid base pair needs to exist in equilibrium. This way, when the pH of the solution changes, the equilibrium of the acid/base reaction will shift, such that the pH will not change drastically.
To have an acid/base pair in equilibrium, we'll need to look for a pair that contains a weak acid. Acids like 



Compare your answer with the correct one above
Consider the following reaction:

Give the expression for the equilibrium constant for this reaction.
Consider the following reaction:
Give the expression for the equilibrium constant for this reaction.
Recall how to find the expression of the equilibrium constant for the simplified equation:

![\text{K}_{eq}=\frac{[C]^c[D]^d}{[A]^a[B]^b}](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/679585/gif.latex)
Since the given equation has gases, we will only consider the partial pressures of each gas in the expression for the equilibrium constant. Remember that only molecules in aqueous and gas forms are included in this expression. Pure solids and pure liquids are excluded.
Thus, we can then write the following equilibrium constant for the given equation:

Recall how to find the expression of the equilibrium constant for the simplified equation:
Since the given equation has gases, we will only consider the partial pressures of each gas in the expression for the equilibrium constant. Remember that only molecules in aqueous and gas forms are included in this expression. Pure solids and pure liquids are excluded.
Thus, we can then write the following equilibrium constant for the given equation:
Compare your answer with the correct one above