Balancing Redox Reactions - College Chemistry
Card 0 of 1
Balance the following redox reaction in acidic conditions:

Balance the following redox reaction in acidic conditions:
Start by writing the half reactions for the equation


All atoms except for O and H are already balanced, so we can go straight to balancing O and H atoms. First balance O atoms by adding H2O
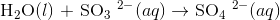

Now balance H atoms with H+


Balance each half reaction's electrical charge with electrons


Now combine both half reactions to get the overall reaction. Be sure to cancel out electrons by multiplying the oxidation reaction by 5 and the reduction reaction by 2. This will give 10 e- on each side of the overall reaction so that they cancel out.


Overall Reaction:


Notice that we have H2O and H+ on both sides of the equation, so we need to simplify. Subtract the 5 H2O on the reactant side from the 8 H2O on the product side which leaves 3 H2O on the reactant side. Then subtract the 10 H+ on the product side from the 16 H+ on the reactant side which leave 6 H+ on the reactant side. Now we have the simplified overall reaction

Start by writing the half reactions for the equation
All atoms except for O and H are already balanced, so we can go straight to balancing O and H atoms. First balance O atoms by adding H2O
Now balance H atoms with H+
Balance each half reaction's electrical charge with electrons
Now combine both half reactions to get the overall reaction. Be sure to cancel out electrons by multiplying the oxidation reaction by 5 and the reduction reaction by 2. This will give 10 e- on each side of the overall reaction so that they cancel out.
Overall Reaction:
Notice that we have H2O and H+ on both sides of the equation, so we need to simplify. Subtract the 5 H2O on the reactant side from the 8 H2O on the product side which leaves 3 H2O on the reactant side. Then subtract the 10 H+ on the product side from the 16 H+ on the reactant side which leave 6 H+ on the reactant side. Now we have the simplified overall reaction
Compare your answer with the correct one above