Thermochemistry and Changes of State
Practice Questions
College Chemistry › Thermochemistry and Changes of State
Which of the following is true of a closed system?
Ice cubes are dropped into a glass of water. You notice that the glass of water becomes colder and condensation appears. Later in the day, you notice the glass of water is now room temperature and there is no more condensation. Which of the following concepts describes this process?
As heat energy is added to a cube of ice, it begins to melt into liquid water. Which of the following correctly identifies the change in temperature and the change in internal energy of the ice as heat is added to it?
Consider the typical phase diagram of a compound given below.
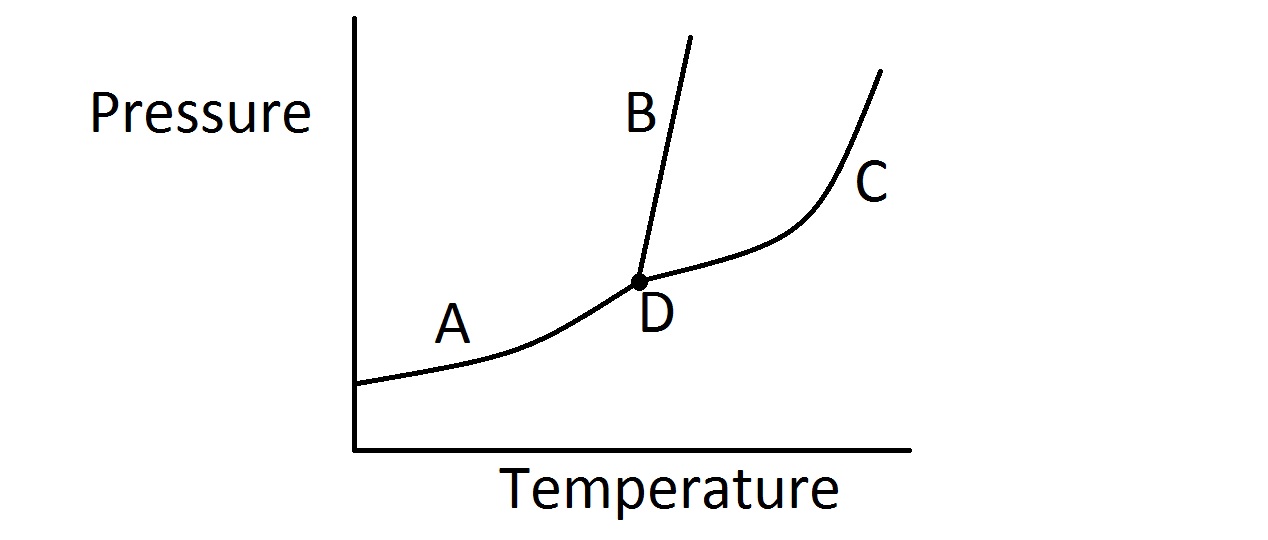
Which of the following lines or points on the diagram represents a situation in which the rate of vaporization of the compound is equal to its rate of condensation?
Use average bond enthalpies to estimate the enthalpy change, in kilojoules, of the combustion of one mole of hexane 
| Type of Bond | Average bond enthalpy  |
|---|---|
| Carbon-hydrogen single bond | 414 |
| Carbon-oxygen double bond | 736 |
| Oxygen-oxygen double bond | 498 |
| Oxygen-hydrogen single bond | 464 |
| Carbon-carbon single bond | 347 |
Ice cubes are dropped into a glass of water. You notice that the glass of water becomes colder and condensation appears. Later in the day, you notice the glass of water is now room temperature and there is no more condensation. Which of the following concepts describes this process?
Suppose a source of water is boiling. If the ambient pressure above the surface of the water were to increase, which of the following would happen to the boiling water?
Consider the typical phase diagram of a compound given below.

Which of the following lines or points on the diagram represents a situation in which the rate of vaporization of the compound is equal to its rate of condensation?
A 



Which of the following is true of a closed system?