Gases
Practice Questions
College Chemistry › Gases
A gas does 


One flask is at STP and another is at 
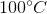
A sample of gas at a constant temperature has an initial pressure of 


A sample of gas at a constant temperature has an initial pressure of 


One flask is at STP and another is at 
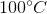
Suppose that hydrochloric acid and aluminum are reacted to produce hydrogen gas. This hydrogen gas is collected by displacement of water at 


Note: The vapor pressure of water at 

A 4.0 L container at 25 C is filled with 2.0 g neon and 8.0 g helium. What is the total pressure of the mixture?
A gas does 


Suppose that hydrochloric acid and aluminum are reacted to produce hydrogen gas. This hydrogen gas is collected by displacement of water at 


Note: The vapor pressure of water at 

A 4.0 L container at 25 C is filled with 2.0 g neon and 8.0 g helium. What is the total pressure of the mixture?