Solutions, States of Matter, and Thermochemistry
Practice Questions
College Chemistry › Solutions, States of Matter, and Thermochemistry
A sample of gas at a constant temperature has an initial pressure of 


Suppose that hydrochloric acid and aluminum are reacted to produce hydrogen gas. This hydrogen gas is collected by displacement of water at 


Note: The vapor pressure of water at 

A 4.0 L container at 25 C is filled with 2.0 g neon and 8.0 g helium. What is the total pressure of the mixture?
Suppose a source of water is boiling. If the ambient pressure above the surface of the water were to increase, which of the following would happen to the boiling water?
Ice cubes are dropped into a glass of water. You notice that the glass of water becomes colder and condensation appears. Later in the day, you notice the glass of water is now room temperature and there is no more condensation. Which of the following concepts describes this process?
Use average bond enthalpies to estimate the enthalpy change, in kilojoules, of the combustion of one mole of hexane 
| Type of Bond | Average bond enthalpy  |
|---|---|
| Carbon-hydrogen single bond | 414 |
| Carbon-oxygen double bond | 736 |
| Oxygen-oxygen double bond | 498 |
| Oxygen-hydrogen single bond | 464 |
| Carbon-carbon single bond | 347 |
A 



Which of the following is true of a closed system?
One flask is at STP and another is at 
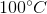
Suppose that hydrochloric acid and aluminum are reacted to produce hydrogen gas. This hydrogen gas is collected by displacement of water at 


Note: The vapor pressure of water at 
