Redox Reactions
Practice Questions
College Chemistry › Redox Reactions
Determine the oxidation number of each element in the compound
Balance the following redox reaction in acidic conditions:
Electrolysis of an unknown metal chloride, 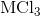
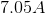
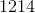
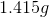
Consider the following redox reaction.
If 




Consider the following redox reaction carried out in a voltaic cell.
If the reduction potential for 






Electrolysis of an unknown metal chloride, 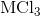
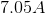
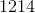
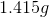
Consider the following redox reaction.
If 




Consider the following redox reaction carried out in a voltaic cell.
If the reduction potential for 






Determine the oxidation number of each element in the compound
Balance the following redox reaction in acidic conditions:



