Protein Structure and Functions - Biochemistry
Card 0 of 20
Which of the following describes induced fit regarding enzyme/substrate binding?
Which of the following describes induced fit regarding enzyme/substrate binding?
The induced fit model explains one method by which an enzyme's active site can accept some specific substrate. Initially, the active site might not be a perfect match for the substrate, however, when the substrate enters into the site, it can change the conformation of the enzyme just enough that it now fits perfectly and can be acted upon by the enzyme.
The induced fit model explains one method by which an enzyme's active site can accept some specific substrate. Initially, the active site might not be a perfect match for the substrate, however, when the substrate enters into the site, it can change the conformation of the enzyme just enough that it now fits perfectly and can be acted upon by the enzyme.
Compare your answer with the correct one above
Suppose that the active site of an enzyme contains amino acid residues at the following positions:
Residue  - Arginine
- Arginine
Residue  - Valine
- Valine
Residue  - Glutamate
- Glutamate
Residue  - Glycine
- Glycine
Which of the following amino acid substitutions would be least likely to affect the activity of this enzyme?
Suppose that the active site of an enzyme contains amino acid residues at the following positions:
Residue 
Residue 
Residue 
Residue 
Which of the following amino acid substitutions would be least likely to affect the activity of this enzyme?
To answer this question, we need to have a general understanding about amino acid properties. For instance, at physiological pH, some amino acid side chains will carry a negative charge, some will carry a positive charge, and others will be neutral. Thus, we'll need to take note of which amino acid characteristics each position has, and then evaluate each answer choice to see if the new amino acid being substituted has different characteristics.
At position  is arginine, which carries a positive charge. At position
is arginine, which carries a positive charge. At position  is valine, which has an aliphatic side chain that is neutral and relatively hydrophobic. At position
is valine, which has an aliphatic side chain that is neutral and relatively hydrophobic. At position  is the amino acid glutamate, which is negatively charged due to the carboxyl group on its side chain. Finally, we have glycine at position
is the amino acid glutamate, which is negatively charged due to the carboxyl group on its side chain. Finally, we have glycine at position  , which contains a lonely hydrogen atom as its side chain.
, which contains a lonely hydrogen atom as its side chain.
Now that we have the characteristics of the amino acid residues in the enzyme, let's compare them to the substitutions listed in the answer choices.
Substituting an aspartate residue into position  would mean replacing valine (neutral) with a positively charged amino acid. Hence, this would likely result in disruption of enzyme activity.
would mean replacing valine (neutral) with a positively charged amino acid. Hence, this would likely result in disruption of enzyme activity.
Substituting a tryptophan residue into position  would replace glycine. In contrast to the extremely small side chain of glycine, the side chain of tryptophan is very large. This great size discrepancy could potentially lead to steric effects that could interfere with the binding of substrate to the enzyme.
would replace glycine. In contrast to the extremely small side chain of glycine, the side chain of tryptophan is very large. This great size discrepancy could potentially lead to steric effects that could interfere with the binding of substrate to the enzyme.
Substitution of an asparagine residue into position  would replace glutamate. Because glutamate is negatively charged, whereas asparagine is neutral, this substitution would likely interfere with enzyme activity.
would replace glutamate. Because glutamate is negatively charged, whereas asparagine is neutral, this substitution would likely interfere with enzyme activity.
Finally, let's consider the substitution of arginine at position  with a lysine. In this case, a positively charged arginine would be replaced by another positively charged amino acid, lysine. Because of the similarity between these two amino acids, this substitution would be the least likely to cause a disruption in the enzyme's activity.
with a lysine. In this case, a positively charged arginine would be replaced by another positively charged amino acid, lysine. Because of the similarity between these two amino acids, this substitution would be the least likely to cause a disruption in the enzyme's activity.
To answer this question, we need to have a general understanding about amino acid properties. For instance, at physiological pH, some amino acid side chains will carry a negative charge, some will carry a positive charge, and others will be neutral. Thus, we'll need to take note of which amino acid characteristics each position has, and then evaluate each answer choice to see if the new amino acid being substituted has different characteristics.
At position 



Now that we have the characteristics of the amino acid residues in the enzyme, let's compare them to the substitutions listed in the answer choices.
Substituting an aspartate residue into position 
Substituting a tryptophan residue into position 
Substitution of an asparagine residue into position 
Finally, let's consider the substitution of arginine at position 
Compare your answer with the correct one above
Which proteins are generally water-soluble?
Which proteins are generally water-soluble?
In a globular protein, the amino acid chain can twist in a way that polar groups lie at the protein's surface. This allows the protein to interact with water and enhances the protein's solubility in water. This does not occur in fibrous proteins, so fibrous proteins are insoluble in water.
In a globular protein, the amino acid chain can twist in a way that polar groups lie at the protein's surface. This allows the protein to interact with water and enhances the protein's solubility in water. This does not occur in fibrous proteins, so fibrous proteins are insoluble in water.
Compare your answer with the correct one above
Which of the following is false about actin filaments?
Which of the following is false about actin filaments?
Actin chain growth occurs at the (+) end of the chain, and nucleotide hydrolysis promotes dissociation of actin chains. 2 microfilaments of G-actin monomers make 1 filament of F-actin. However, actin filament assembly is powered by ATP, not GTP.
Actin chain growth occurs at the (+) end of the chain, and nucleotide hydrolysis promotes dissociation of actin chains. 2 microfilaments of G-actin monomers make 1 filament of F-actin. However, actin filament assembly is powered by ATP, not GTP.
Compare your answer with the correct one above
An O-linked glycoprotein has a sugar attached to an oxygen atom on what amino acid(s)?
An O-linked glycoprotein has a sugar attached to an oxygen atom on what amino acid(s)?
An O-linked glycoprotein is a protein that has a sugar attached to it. It is called O-linked because the sugar is attached to an oxygen atom on either a threonine residue or a serine residue within the protein.
An O-linked glycoprotein is a protein that has a sugar attached to it. It is called O-linked because the sugar is attached to an oxygen atom on either a threonine residue or a serine residue within the protein.
Compare your answer with the correct one above
Lysosomal enzymes are predominantly __________.
Lysosomal enzymes are predominantly __________.
The lysosome is the "stomach" of the cell. It contains many hydrolytic enzymes to digest and recycle the monomers used to form old polymers. Remember the opposite of dehydration/condensation synthesis is hydrolysis. Hydrolysis reactions use water to break bonds in polymers, yielding monomers that can be recycled and reused in anabolic pathways.
The lysosome is the "stomach" of the cell. It contains many hydrolytic enzymes to digest and recycle the monomers used to form old polymers. Remember the opposite of dehydration/condensation synthesis is hydrolysis. Hydrolysis reactions use water to break bonds in polymers, yielding monomers that can be recycled and reused in anabolic pathways.
Compare your answer with the correct one above
Which of the following best describes how a lysozyme works?
Which of the following best describes how a lysozyme works?
Lysozymes speed up by many times the hydrolysis of polysaccharides, by adding the water molecule to sugars linked in its enzyme-substrate complex. If left alone without the lysozyme, this hydrolysis would occur relatively infrequently, because it requires a large activation energy which would be supplied only by rare random collisions. The amino acid cleavage enzyme which uses the ping-pong mechanism is chymotrypsin. The enzyme which breaks nucleic acid phosophodiester bonds is phosphodiesterase. Fats are hydrolyzed by lipases, not lysozymes.
Lysozymes speed up by many times the hydrolysis of polysaccharides, by adding the water molecule to sugars linked in its enzyme-substrate complex. If left alone without the lysozyme, this hydrolysis would occur relatively infrequently, because it requires a large activation energy which would be supplied only by rare random collisions. The amino acid cleavage enzyme which uses the ping-pong mechanism is chymotrypsin. The enzyme which breaks nucleic acid phosophodiester bonds is phosphodiesterase. Fats are hydrolyzed by lipases, not lysozymes.
Compare your answer with the correct one above
Phosphoglucomutase is an enzyme seen in glycogen breakdown. It is responsible for converting glucose-1-phosphate ( ) to glucose-6-phosphate (
) to glucose-6-phosphate (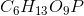 ).
).
Based on this action, to which enzyme class does phosphoglucomutase belong?
Phosphoglucomutase is an enzyme seen in glycogen breakdown. It is responsible for converting glucose-1-phosphate (
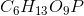
Based on this action, to which enzyme class does phosphoglucomutase belong?
Phosphoglucomutase is responsible for altering the position of the phosphate on the glucose from the "1" position to the "6" position. However, notice how the molecular formula for the product and the substrate are the same. Enzymes that rearrange the structure of a molecule in this manner are referred to as isomerase enzymes.
Phosphoglucomutase is responsible for altering the position of the phosphate on the glucose from the "1" position to the "6" position. However, notice how the molecular formula for the product and the substrate are the same. Enzymes that rearrange the structure of a molecule in this manner are referred to as isomerase enzymes.
Compare your answer with the correct one above
Which of the following correctly mentions the function of a common eukaryotic ligase?
Which of the following correctly mentions the function of a common eukaryotic ligase?
Mammalian DNA ligase I has this function, and there are other DNA ligases which perform it in other animals and eukaryotes (prokaryotes also have their own DNA ligases). All the other functions mentioned are done by other classes of enzymes, not ligases (i.e. hydrolases, aminotransferases, oxidoreductases, etc.).
Mammalian DNA ligase I has this function, and there are other DNA ligases which perform it in other animals and eukaryotes (prokaryotes also have their own DNA ligases). All the other functions mentioned are done by other classes of enzymes, not ligases (i.e. hydrolases, aminotransferases, oxidoreductases, etc.).
Compare your answer with the correct one above
Hemoglobin is an important component of red blood cells that allows for efficient delivery of oxygen from the lungs to tissues throughout the body. Without hemoglobin, oxygen would not be able to dissolve in the blood well enough to meet the metabolic needs of tissues. Hemoglobin is well-suited to this function because its affinity for oxygen varies depending on its chemical environment. Which of the following situations would cause a rightward shift on the oxygen dissociation curve for hemoglobin?
Hemoglobin is an important component of red blood cells that allows for efficient delivery of oxygen from the lungs to tissues throughout the body. Without hemoglobin, oxygen would not be able to dissolve in the blood well enough to meet the metabolic needs of tissues. Hemoglobin is well-suited to this function because its affinity for oxygen varies depending on its chemical environment. Which of the following situations would cause a rightward shift on the oxygen dissociation curve for hemoglobin?
An oxygen dissociation curve for hemoglobin plots the percent saturation of oxygen on the  -axis vs. the partial pressure of oxygen on the
-axis vs. the partial pressure of oxygen on the  -axis. A rightward shift of the curve means that for a given oxygen saturation level, there needs to be a higher partial pressure of oxygen. Thus, a rightward shift is indicative of a decreased affinity of hemoglobin for oxygen.
-axis. A rightward shift of the curve means that for a given oxygen saturation level, there needs to be a higher partial pressure of oxygen. Thus, a rightward shift is indicative of a decreased affinity of hemoglobin for oxygen.
There are several factors that can influence hemoglobin's affinity for oxygen. One such factor is pH. At lower pH levels, hemoglobin has a more difficult time holding onto oxygen. Physiologically this makes sense, because the blood is likely to be slightly more acidic in regions where tissues are metabolically active, hence they are going to need more oxygen to sustain their metabolism. Likewise, carbon dioxide is also capable of lowering hemoglobin's affinity for oxygen. And again, this makes sense physiologically, because tissues with a high metabolism are going to be generating more carbon dioxide, which serves as a signal to allow hemoglobin to drop off more oxygen for these active tissues. And finally, an additional regulatory factor is a glycolytic intermediate called 2,3-bisphosphoglycerate (2,3-BPG). Binding of this compound to hemoglobin lowers oxygen affinity, thus higher concentrations of 2,3-BPG also cause a rightward shift of the curve.
Also note that oxygen binds hemoglobin in a cooperative fashion. This means that when one molecule of oxygen binds to hemoglobin, the other three oxygen binding sites on hemoglobin gain subsequently increased affinity for oxygen. And when a second oxygen molecule binds, the other binding sites gain more affinity, and so on. Thus, when the partial pressure of oxygen increases, hemoglobin's affinity for oxygen becomes greater.
An oxygen dissociation curve for hemoglobin plots the percent saturation of oxygen on the 

There are several factors that can influence hemoglobin's affinity for oxygen. One such factor is pH. At lower pH levels, hemoglobin has a more difficult time holding onto oxygen. Physiologically this makes sense, because the blood is likely to be slightly more acidic in regions where tissues are metabolically active, hence they are going to need more oxygen to sustain their metabolism. Likewise, carbon dioxide is also capable of lowering hemoglobin's affinity for oxygen. And again, this makes sense physiologically, because tissues with a high metabolism are going to be generating more carbon dioxide, which serves as a signal to allow hemoglobin to drop off more oxygen for these active tissues. And finally, an additional regulatory factor is a glycolytic intermediate called 2,3-bisphosphoglycerate (2,3-BPG). Binding of this compound to hemoglobin lowers oxygen affinity, thus higher concentrations of 2,3-BPG also cause a rightward shift of the curve.
Also note that oxygen binds hemoglobin in a cooperative fashion. This means that when one molecule of oxygen binds to hemoglobin, the other three oxygen binding sites on hemoglobin gain subsequently increased affinity for oxygen. And when a second oxygen molecule binds, the other binding sites gain more affinity, and so on. Thus, when the partial pressure of oxygen increases, hemoglobin's affinity for oxygen becomes greater.
Compare your answer with the correct one above
Which of the following statements about ribosomes is false?
Which of the following statements about ribosomes is false?
Ribozymes are RNA molecules that catalyze specific biochemical reactions, so ribosomes (which catalyze the linking of amino acids) are indeed ribozymes. tRNA is complementary to ribosomal RNA in the sites where the two bind. Aminoacylation produces a tRNA with its 3’ end covalently linked to an amino acid. The sequence at the 3’ end is not ACA, however’ it is CCA.
Ribozymes are RNA molecules that catalyze specific biochemical reactions, so ribosomes (which catalyze the linking of amino acids) are indeed ribozymes. tRNA is complementary to ribosomal RNA in the sites where the two bind. Aminoacylation produces a tRNA with its 3’ end covalently linked to an amino acid. The sequence at the 3’ end is not ACA, however’ it is CCA.
Compare your answer with the correct one above
What term is used to describe enzymes that have different chemical structures but which catalyze the same reactions?
What term is used to describe enzymes that have different chemical structures but which catalyze the same reactions?
The correct answer choice is isozymes, also called isoenzymes. Even though these enzymes can catalyze the same reaction, they often have differences in their kinetic parameters or in the way they're regulated. Coenzymes are a type of cofactor. They are generally complex organic molecules that are usually derived from vitamins, and they serve the purpose of assisting the enzyme to which they are bound. Examples include pyridoxal phosphate, biotin, coenzyme A, etc. Apoenzymes are enzymes that normally require a cofactor, but are in a state in which they lack that cofactor. Holoenzymes are apoenzymes that have their cofactor bound.
The correct answer choice is isozymes, also called isoenzymes. Even though these enzymes can catalyze the same reaction, they often have differences in their kinetic parameters or in the way they're regulated. Coenzymes are a type of cofactor. They are generally complex organic molecules that are usually derived from vitamins, and they serve the purpose of assisting the enzyme to which they are bound. Examples include pyridoxal phosphate, biotin, coenzyme A, etc. Apoenzymes are enzymes that normally require a cofactor, but are in a state in which they lack that cofactor. Holoenzymes are apoenzymes that have their cofactor bound.
Compare your answer with the correct one above
Which of the following is true about chromoproteins?
Which of the following is true about chromoproteins?
Heme normally binds to iron. Myoglobin is mostly concentrated in muscles, and after a muscle injury can be present in blood. Myoglobin has a higher affinity for oxygen than hemoglobin; myoglobin’s oxygen saturation curve is hyperbolic, whereas hemoglobin’s is sigmoidal. Hemoglobin F (fetal hemoglobin) has a higher oxygen affinity than hemoglobin A (adult hemoglobin). This improves the transfer of oxygen from the circulation of the mother to that of the fetus.
Heme normally binds to iron. Myoglobin is mostly concentrated in muscles, and after a muscle injury can be present in blood. Myoglobin has a higher affinity for oxygen than hemoglobin; myoglobin’s oxygen saturation curve is hyperbolic, whereas hemoglobin’s is sigmoidal. Hemoglobin F (fetal hemoglobin) has a higher oxygen affinity than hemoglobin A (adult hemoglobin). This improves the transfer of oxygen from the circulation of the mother to that of the fetus.
Compare your answer with the correct one above
Which of the following is not a characteristic of chymotrypsin?
Which of the following is not a characteristic of chymotrypsin?
Chymotrypsin is a digestive enzyme that breaks down proteins (proteolysis). It has a catalytic triad of serine, histidine, and aspartate. The hydroxyl group on serine acts as a nucleophile and attacks the carbonyl group on the amino acid, forming a tetrahedral intermediate. Histidine acts as a base, which cleaves the peptide bond. Aspartate acts as an acid, which restores the active site. Since this catalytic triad has a defined nucleophile, base, and acid, we know that there will not be an additional thiol nucleophile. Thiol nucleophiles are found in cysteine proteases.
Chymotrypsin is a digestive enzyme that breaks down proteins (proteolysis). It has a catalytic triad of serine, histidine, and aspartate. The hydroxyl group on serine acts as a nucleophile and attacks the carbonyl group on the amino acid, forming a tetrahedral intermediate. Histidine acts as a base, which cleaves the peptide bond. Aspartate acts as an acid, which restores the active site. Since this catalytic triad has a defined nucleophile, base, and acid, we know that there will not be an additional thiol nucleophile. Thiol nucleophiles are found in cysteine proteases.
Compare your answer with the correct one above
Generally speaking, proteins have many important functions in living organisms. Which of the following is not a potential function of proteins?
Generally speaking, proteins have many important functions in living organisms. Which of the following is not a potential function of proteins?
As stated in the question stem, proteins are super important because they perform an enormous variety of functions. Proteins can sometimes serve a structural role, such as the protein collagen which helps give some connective tissues their unique properties. They can also act as chemical messengers, as some protein hormones do such as growth hormone. Some proteins, such as antibodies, can even protect against infection by neutralizing invading pathogens such as bacteria. In addition, proteins can also exist as enzymes, which act to greatly increase the rate of specific reactions. Other proteins can act as carriers or transporters that help to facilitate the movement of some chemicals across an otherwise impermeable cell membrane. While it's true that proteins play a very important structural and functional role in the cell membrane, they are not the primary structural component. Rather, phospholipids serve as the most abundant component of cell membranes and helps to give them their unique role as "cellular barriers."
As stated in the question stem, proteins are super important because they perform an enormous variety of functions. Proteins can sometimes serve a structural role, such as the protein collagen which helps give some connective tissues their unique properties. They can also act as chemical messengers, as some protein hormones do such as growth hormone. Some proteins, such as antibodies, can even protect against infection by neutralizing invading pathogens such as bacteria. In addition, proteins can also exist as enzymes, which act to greatly increase the rate of specific reactions. Other proteins can act as carriers or transporters that help to facilitate the movement of some chemicals across an otherwise impermeable cell membrane. While it's true that proteins play a very important structural and functional role in the cell membrane, they are not the primary structural component. Rather, phospholipids serve as the most abundant component of cell membranes and helps to give them their unique role as "cellular barriers."
Compare your answer with the correct one above
Which of these describes the protein myoglobin?
Which of these describes the protein myoglobin?
Myoglobin and hemoglobin are not the same molecule; their functions are similar but different in several ways. Myoglobin is an oxygen-binding protein of muscle tissues. In contrast, hemoglobin is the oxygen-transport protein found in blood. Hemoglobin can bind four oxygen atoms, while myoglobin can only bind one. Both hemoglobin and myoglobin contain heme groups, with hemoglobin containing four and myoglobin containing one. These iron-containing groups are responsible for binding the oxygen atom(s).
Myoglobin and hemoglobin are not the same molecule; their functions are similar but different in several ways. Myoglobin is an oxygen-binding protein of muscle tissues. In contrast, hemoglobin is the oxygen-transport protein found in blood. Hemoglobin can bind four oxygen atoms, while myoglobin can only bind one. Both hemoglobin and myoglobin contain heme groups, with hemoglobin containing four and myoglobin containing one. These iron-containing groups are responsible for binding the oxygen atom(s).
Compare your answer with the correct one above
Which of these is not a function of membrane proteins?
Which of these is not a function of membrane proteins?
Membrane proteins have several functions. They can act as catalysts, receptor proteins for different molecules attempting to enter/exit the cell, and also function in transport as channels or transporters. However, energy storage is a function that is carried out by carbohydrates and lipids.
Membrane proteins have several functions. They can act as catalysts, receptor proteins for different molecules attempting to enter/exit the cell, and also function in transport as channels or transporters. However, energy storage is a function that is carried out by carbohydrates and lipids.
Compare your answer with the correct one above
Which of the following correctly describes an allosteric enzyme?
Which of the following correctly describes an allosteric enzyme?
Allosteric enzymes are enzymes that can be regulated by binding of a small molecule to an "allosteric site" on the enzyme. This is a location on the enzyme that is distinct from the active site, which is where the enzyme binds to its substrate.
Allosteric compounds can be either activators or repressors. Activators cause the enzyme's activity to increase, whereas repressors cause the enzyme's activity to decrease. This happens because binding of an allosteric molecule acts to change the enzyme's structural conformation, even if just slightly, which thus makes it either more active or less active.
For example, phosphofructokinase is an enzyme found in glycolysis, the metabolic pathway that breaks glucose down in cells for energy. This enzyme is a critical component of the pathway, because it is largely responsible for regulating the flux of glucose through this pathway. As such, there are many cellular metabolites that act to either increase or decrease this enzyme's activity. As an example, molecules like ATP and other intermediates of glycolysis and, subsequently, the citric acid cycle are able to decrease this enzyme's activity. Some of these metabolites include phosphoenolpyruvate and citric acid, in addition to ATP, all of which indicate that the cell has an abundant amount of energy available. AMP, on the other hand, acts to allosterically activate phosphofructokinase because it signals that the cell is low in energy. Thus, allosteric enzymes are an important component of how biochemical processes can be regulated.
Allosteric enzymes are enzymes that can be regulated by binding of a small molecule to an "allosteric site" on the enzyme. This is a location on the enzyme that is distinct from the active site, which is where the enzyme binds to its substrate.
Allosteric compounds can be either activators or repressors. Activators cause the enzyme's activity to increase, whereas repressors cause the enzyme's activity to decrease. This happens because binding of an allosteric molecule acts to change the enzyme's structural conformation, even if just slightly, which thus makes it either more active or less active.
For example, phosphofructokinase is an enzyme found in glycolysis, the metabolic pathway that breaks glucose down in cells for energy. This enzyme is a critical component of the pathway, because it is largely responsible for regulating the flux of glucose through this pathway. As such, there are many cellular metabolites that act to either increase or decrease this enzyme's activity. As an example, molecules like ATP and other intermediates of glycolysis and, subsequently, the citric acid cycle are able to decrease this enzyme's activity. Some of these metabolites include phosphoenolpyruvate and citric acid, in addition to ATP, all of which indicate that the cell has an abundant amount of energy available. AMP, on the other hand, acts to allosterically activate phosphofructokinase because it signals that the cell is low in energy. Thus, allosteric enzymes are an important component of how biochemical processes can be regulated.
Compare your answer with the correct one above
Which of the following reaction types is not catalyzed by cobalamin enzymes?
Which of the following reaction types is not catalyzed by cobalamin enzymes?
Cobalamin enzymes are B12-dependent enzymes. This type of enzyme catalyzes methylations, intramolecular rearrangements, and reduction of ribonucleotides. However, it is not associated with the ligation of the hydrogen bonds between DNA bases.
Cobalamin enzymes are B12-dependent enzymes. This type of enzyme catalyzes methylations, intramolecular rearrangements, and reduction of ribonucleotides. However, it is not associated with the ligation of the hydrogen bonds between DNA bases.
Compare your answer with the correct one above
Where does steroid synthesis and detoxification of drugs and poisons occur in a cell?
Where does steroid synthesis and detoxification of drugs and poisons occur in a cell?
Liver hepatocytes and steroid hormone producing cells of the adrenal cortex are rich in the SER. RER is the site of synthesis of secretory (exported) proteins. The golgi apparatus does many things, but in general, think of it as the distribution center and vesicular trafficking. It organizes and directs where everything should go. The nucleolus is unrelated to this topic, but it does produce ribosomes.
Liver hepatocytes and steroid hormone producing cells of the adrenal cortex are rich in the SER. RER is the site of synthesis of secretory (exported) proteins. The golgi apparatus does many things, but in general, think of it as the distribution center and vesicular trafficking. It organizes and directs where everything should go. The nucleolus is unrelated to this topic, but it does produce ribosomes.
Compare your answer with the correct one above