Photoelectron Spectroscopy - AP Chemistry
Card 0 of 15
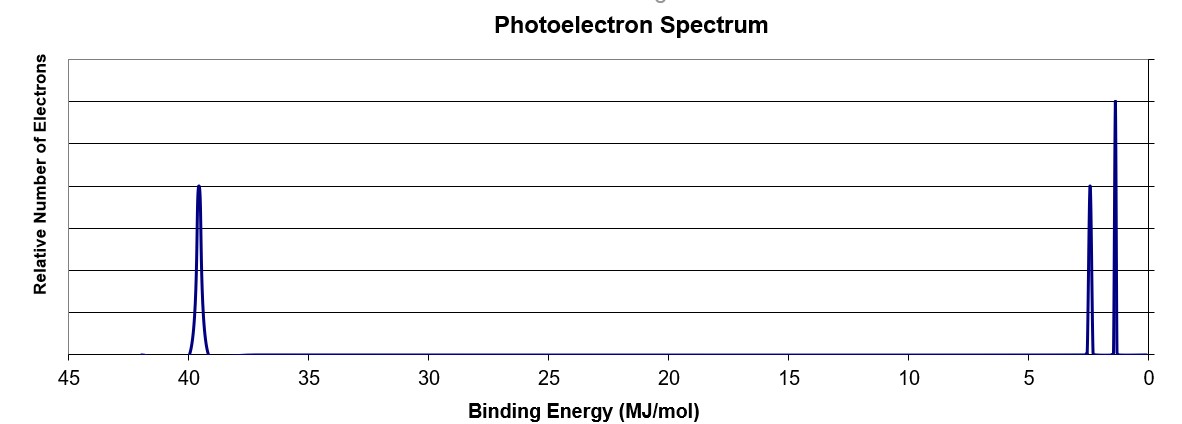
Using the PES spectra above, what element is illustrated?

Using the PES spectra above, what element is illustrated?
The peak at ~ 40 MJ binding energy corresponds to the 1s orbital. Since there are peaks at lower binding energy, it is implied that this orbital is filled (by 2 electrons). There are two peaks at lower binding energy corresponding to the 2s and 2p orbitals, respectively. The 2s peak has the same intensity as the 1s peak indicating it has the same number of electrons (2). The 2p peak is 3/2 as tall as the 2s peak and indicates that indicates it has 3 electrons in the orbital. The electron configuration of 1s22s22p3 makes the element N.
The peak at ~ 40 MJ binding energy corresponds to the 1s orbital. Since there are peaks at lower binding energy, it is implied that this orbital is filled (by 2 electrons). There are two peaks at lower binding energy corresponding to the 2s and 2p orbitals, respectively. The 2s peak has the same intensity as the 1s peak indicating it has the same number of electrons (2). The 2p peak is 3/2 as tall as the 2s peak and indicates that indicates it has 3 electrons in the orbital. The electron configuration of 1s22s22p3 makes the element N.
Compare your answer with the correct one above

Using the PES spectra above, what answer explains the differences in the position and intensity of the 3s peaks between Na and Mg?

Using the PES spectra above, what answer explains the differences in the position and intensity of the 3s peaks between Na and Mg?
Mg has a Z=12 while Na is Z=11. The increased number of protons in Mg gives rise to a greater effective nuclear charge. This greater effective nuclear charge binds the electrons in their respective orbitals more tightly than an element with a lower effective nuclear charge. The peak height of Mg is 2x that of Na for the 3s orbital because it has twice the electrons present.
Mg has a Z=12 while Na is Z=11. The increased number of protons in Mg gives rise to a greater effective nuclear charge. This greater effective nuclear charge binds the electrons in their respective orbitals more tightly than an element with a lower effective nuclear charge. The peak height of Mg is 2x that of Na for the 3s orbital because it has twice the electrons present.
Compare your answer with the correct one above

Using the spectra above, answer the following: Why is the oxygen 1s electrons further to the right than the nitrogen 1s orbital?

Using the spectra above, answer the following: Why is the oxygen 1s electrons further to the right than the nitrogen 1s orbital?
Oxygen has a Z=8 vs nitrogen’s Z=7. This greater effective nuclear charge binds the electrons in their respective orbitals more tightly than an element with a lower effective nuclear charge.
Oxygen has a Z=8 vs nitrogen’s Z=7. This greater effective nuclear charge binds the electrons in their respective orbitals more tightly than an element with a lower effective nuclear charge.
Compare your answer with the correct one above
Using the spectra above, answer the following: What is the electron configuration of the element shown above?
Using the spectra above, answer the following: What is the electron configuration of the element shown above?
The binding energy at ~ 53 Mj represents the 1s orbital. With other peaks present, this 1s orbital is fully occupied by 2 electrons. The peaks at lower energy would correspond to the 2s and 2p, respectively. Since the 2p peak is twice the intensity of the 2s or 1_s_ peak, it has twice the electrons (4). This gives the electron configuration 1s22s22p4.
The binding energy at ~ 53 Mj represents the 1s orbital. With other peaks present, this 1s orbital is fully occupied by 2 electrons. The peaks at lower energy would correspond to the 2s and 2p, respectively. Since the 2p peak is twice the intensity of the 2s or 1_s_ peak, it has twice the electrons (4). This gives the electron configuration 1s22s22p4.
Compare your answer with the correct one above

Using the spectra above, answer the following: What is the wavelength required, in m, to remove a valence electron from the element shown above?

Using the spectra above, answer the following: What is the wavelength required, in m, to remove a valence electron from the element shown above?
Step 1. Convert the Binding Energy from MJ/mol to J
 x
x  x
x 
Step 2:


Step 1. Convert the Binding Energy from MJ/mol to J


Step 2:
Compare your answer with the correct one above

Using the PES spectra above, what element is illustrated?

Using the PES spectra above, what element is illustrated?
The peak at ~ 40 MJ binding energy corresponds to the 1s orbital. Since there are peaks at lower binding energy, it is implied that this orbital is filled (by 2 electrons). There are two peaks at lower binding energy corresponding to the 2s and 2p orbitals, respectively. The 2s peak has the same intensity as the 1s peak indicating it has the same number of electrons (2). The 2p peak is 3/2 as tall as the 2s peak and indicates that indicates it has 3 electrons in the orbital. The electron configuration of 1s22s22p3 makes the element N.
The peak at ~ 40 MJ binding energy corresponds to the 1s orbital. Since there are peaks at lower binding energy, it is implied that this orbital is filled (by 2 electrons). There are two peaks at lower binding energy corresponding to the 2s and 2p orbitals, respectively. The 2s peak has the same intensity as the 1s peak indicating it has the same number of electrons (2). The 2p peak is 3/2 as tall as the 2s peak and indicates that indicates it has 3 electrons in the orbital. The electron configuration of 1s22s22p3 makes the element N.
Compare your answer with the correct one above

Using the PES spectra above, what answer explains the differences in the position and intensity of the 3s peaks between Na and Mg?

Using the PES spectra above, what answer explains the differences in the position and intensity of the 3s peaks between Na and Mg?
Mg has a Z=12 while Na is Z=11. The increased number of protons in Mg gives rise to a greater effective nuclear charge. This greater effective nuclear charge binds the electrons in their respective orbitals more tightly than an element with a lower effective nuclear charge. The peak height of Mg is 2x that of Na for the 3s orbital because it has twice the electrons present.
Mg has a Z=12 while Na is Z=11. The increased number of protons in Mg gives rise to a greater effective nuclear charge. This greater effective nuclear charge binds the electrons in their respective orbitals more tightly than an element with a lower effective nuclear charge. The peak height of Mg is 2x that of Na for the 3s orbital because it has twice the electrons present.
Compare your answer with the correct one above

Using the spectra above, answer the following: Why is the oxygen 1s electrons further to the right than the nitrogen 1s orbital?

Using the spectra above, answer the following: Why is the oxygen 1s electrons further to the right than the nitrogen 1s orbital?
Oxygen has a Z=8 vs nitrogen’s Z=7. This greater effective nuclear charge binds the electrons in their respective orbitals more tightly than an element with a lower effective nuclear charge.
Oxygen has a Z=8 vs nitrogen’s Z=7. This greater effective nuclear charge binds the electrons in their respective orbitals more tightly than an element with a lower effective nuclear charge.
Compare your answer with the correct one above
Using the spectra above, answer the following: What is the electron configuration of the element shown above?
Using the spectra above, answer the following: What is the electron configuration of the element shown above?
The binding energy at ~ 53 Mj represents the 1s orbital. With other peaks present, this 1s orbital is fully occupied by 2 electrons. The peaks at lower energy would correspond to the 2s and 2p, respectively. Since the 2p peak is twice the intensity of the 2s or 1_s_ peak, it has twice the electrons (4). This gives the electron configuration 1s22s22p4.
The binding energy at ~ 53 Mj represents the 1s orbital. With other peaks present, this 1s orbital is fully occupied by 2 electrons. The peaks at lower energy would correspond to the 2s and 2p, respectively. Since the 2p peak is twice the intensity of the 2s or 1_s_ peak, it has twice the electrons (4). This gives the electron configuration 1s22s22p4.
Compare your answer with the correct one above

Using the spectra above, answer the following: What is the wavelength required, in m, to remove a valence electron from the element shown above?

Using the spectra above, answer the following: What is the wavelength required, in m, to remove a valence electron from the element shown above?
Step 1. Convert the Binding Energy from MJ/mol to J
 x
x  x
x 
Step 2:


Step 1. Convert the Binding Energy from MJ/mol to J


Step 2:
Compare your answer with the correct one above

Using the PES spectra above, what element is illustrated?

Using the PES spectra above, what element is illustrated?
The peak at ~ 40 MJ binding energy corresponds to the 1s orbital. Since there are peaks at lower binding energy, it is implied that this orbital is filled (by 2 electrons). There are two peaks at lower binding energy corresponding to the 2s and 2p orbitals, respectively. The 2s peak has the same intensity as the 1s peak indicating it has the same number of electrons (2). The 2p peak is 3/2 as tall as the 2s peak and indicates that indicates it has 3 electrons in the orbital. The electron configuration of 1s22s22p3 makes the element N.
The peak at ~ 40 MJ binding energy corresponds to the 1s orbital. Since there are peaks at lower binding energy, it is implied that this orbital is filled (by 2 electrons). There are two peaks at lower binding energy corresponding to the 2s and 2p orbitals, respectively. The 2s peak has the same intensity as the 1s peak indicating it has the same number of electrons (2). The 2p peak is 3/2 as tall as the 2s peak and indicates that indicates it has 3 electrons in the orbital. The electron configuration of 1s22s22p3 makes the element N.
Compare your answer with the correct one above

Using the PES spectra above, what answer explains the differences in the position and intensity of the 3s peaks between Na and Mg?

Using the PES spectra above, what answer explains the differences in the position and intensity of the 3s peaks between Na and Mg?
Mg has a Z=12 while Na is Z=11. The increased number of protons in Mg gives rise to a greater effective nuclear charge. This greater effective nuclear charge binds the electrons in their respective orbitals more tightly than an element with a lower effective nuclear charge. The peak height of Mg is 2x that of Na for the 3s orbital because it has twice the electrons present.
Mg has a Z=12 while Na is Z=11. The increased number of protons in Mg gives rise to a greater effective nuclear charge. This greater effective nuclear charge binds the electrons in their respective orbitals more tightly than an element with a lower effective nuclear charge. The peak height of Mg is 2x that of Na for the 3s orbital because it has twice the electrons present.
Compare your answer with the correct one above

Using the spectra above, answer the following: Why is the oxygen 1s electrons further to the right than the nitrogen 1s orbital?

Using the spectra above, answer the following: Why is the oxygen 1s electrons further to the right than the nitrogen 1s orbital?
Oxygen has a Z=8 vs nitrogen’s Z=7. This greater effective nuclear charge binds the electrons in their respective orbitals more tightly than an element with a lower effective nuclear charge.
Oxygen has a Z=8 vs nitrogen’s Z=7. This greater effective nuclear charge binds the electrons in their respective orbitals more tightly than an element with a lower effective nuclear charge.
Compare your answer with the correct one above
Using the spectra above, answer the following: What is the electron configuration of the element shown above?
Using the spectra above, answer the following: What is the electron configuration of the element shown above?
The binding energy at ~ 53 Mj represents the 1s orbital. With other peaks present, this 1s orbital is fully occupied by 2 electrons. The peaks at lower energy would correspond to the 2s and 2p, respectively. Since the 2p peak is twice the intensity of the 2s or 1_s_ peak, it has twice the electrons (4). This gives the electron configuration 1s22s22p4.
The binding energy at ~ 53 Mj represents the 1s orbital. With other peaks present, this 1s orbital is fully occupied by 2 electrons. The peaks at lower energy would correspond to the 2s and 2p, respectively. Since the 2p peak is twice the intensity of the 2s or 1_s_ peak, it has twice the electrons (4). This gives the electron configuration 1s22s22p4.
Compare your answer with the correct one above

Using the spectra above, answer the following: What is the wavelength required, in m, to remove a valence electron from the element shown above?

Using the spectra above, answer the following: What is the wavelength required, in m, to remove a valence electron from the element shown above?
Step 1. Convert the Binding Energy from MJ/mol to J
 x
x  x
x 
Step 2:


Step 1. Convert the Binding Energy from MJ/mol to J


Step 2:
Compare your answer with the correct one above


