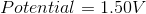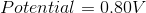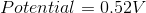Reduction Potential
Practice Questions
AP Chemistry › Reduction Potential
Questions
3
1
The standard reduction potentials for certain metals are listed below:
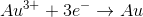




What is the standard potential of a cell in which copper is reduced by iron?
2
Standard Reduction Potentials
Cr3+(aq) + 3e– → Cr (s) –0.74 V
Cu2+(aq) +2e– → Cu (s) 0.34 V
Consider the following reaction
Cu(s) + Cr3+(aq) ⇌ Cu2+(aq) + Cr(s)
What is the Eo Cell for the reaction?
3
The standard reduction potentials for some metals are listed below:





Which of the following metals is the strongest reducing agent?