Photoelectron Spectroscopy
Practice Questions
AP Chemistry › Photoelectron Spectroscopy
Questions
5
1
Using the spectra above, answer the following: What is the electron configuration of the element shown above?
2

Using the spectra above, answer the following: What is the wavelength required, in m, to remove a valence electron from the element shown above?
3

Using the PES spectra above, what answer explains the differences in the position and intensity of the 3s peaks between Na and Mg?
4
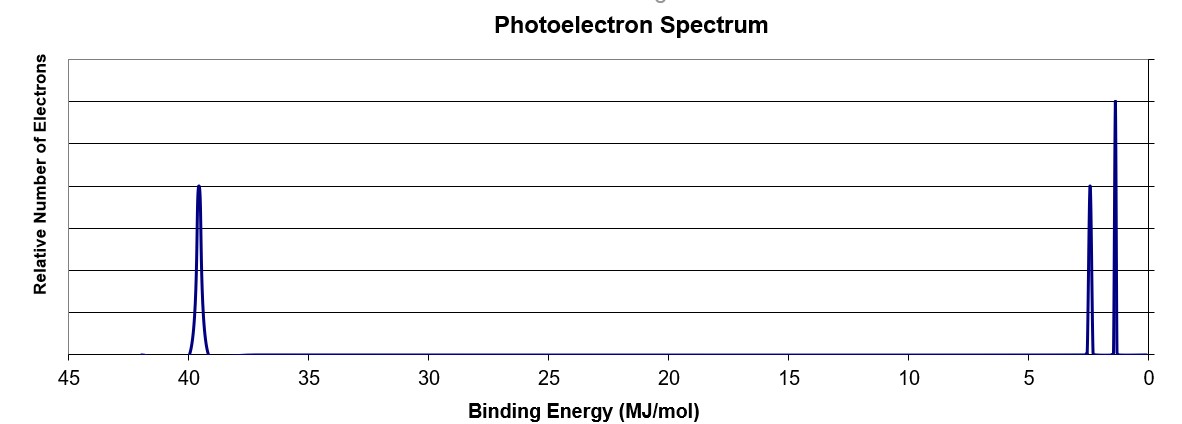
Using the PES spectra above, what element is illustrated?
5

Using the spectra above, answer the following: Why is the oxygen 1s electrons further to the right than the nitrogen 1s orbital?